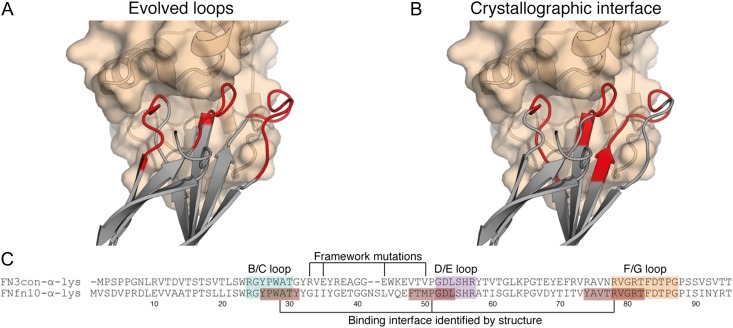
Fig. 2.
The FNfn10-α-lys-lysozyme complex reveals a tight binding interface that makes use of framework residues. (A) The loop residues (red) of FNfn10-α-lys (gray) as previously evolved for lysozyme (tan) binding (Hackel et al., 2008). (B) The actual binding interface residues (red) of FNfn10-α-lys (gray) with lysozyme (tan) as determined by crystal structure and the PDBePISA web server (Krissinel and Henrick, 2007). (C) A sequence alignment of FN3con-α-lys with FNfn10-α-lys, highlighting the B/C, D/E and F/G loops (blue, purple and orange) that were previously evolved for lysozyme binding (Hackel et al., 2008), the actual residues involved in the binding interface (red) and positions of the FNfn10-α-lys framework mutations previously introduced (Hackel et al., 2008).