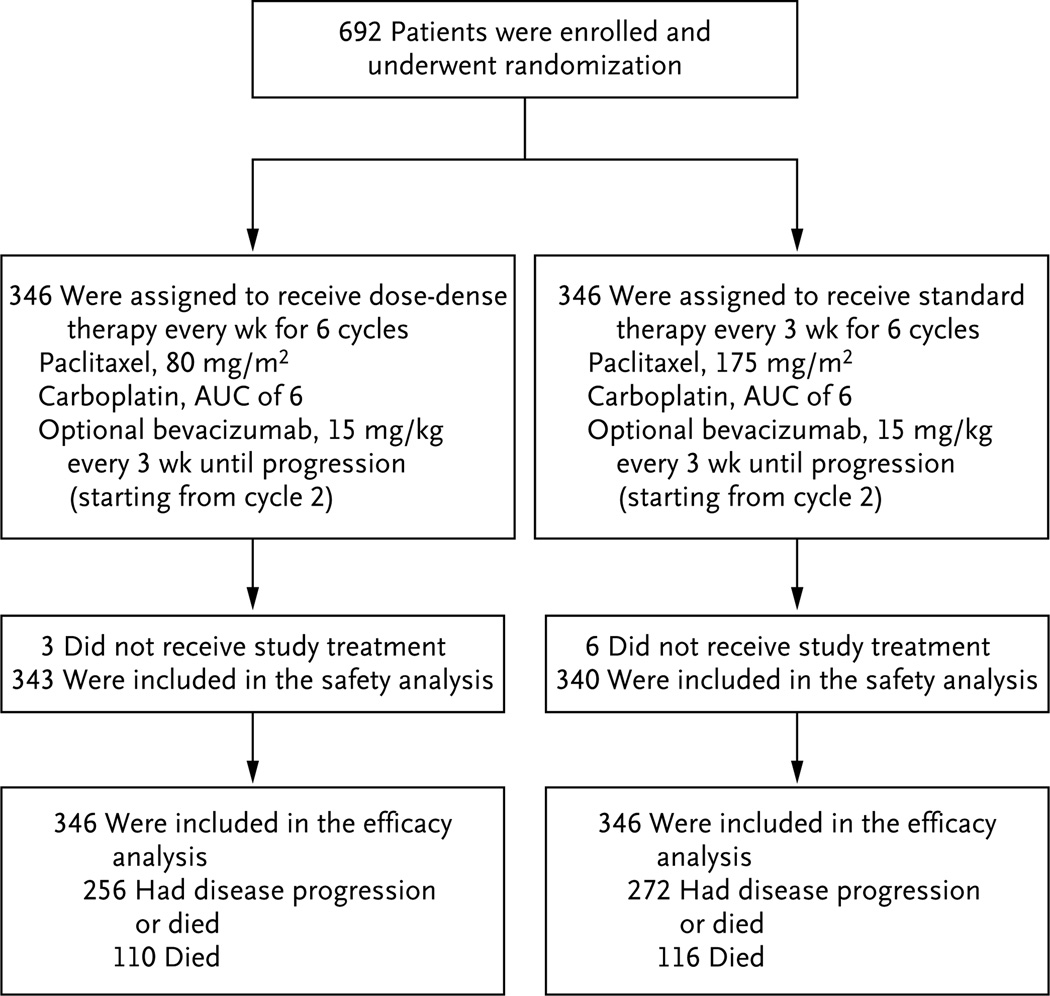
Figure 1. Enrollment, Randomization, and Follow-up of the Study Patients.
Patients who were randomly assigned to dose-dense therapy received paclitaxel at a dose of 80 mg per square meter of body-surface area on days 1, 8, and 15 of a 21-day cycle, plus a carboplatin dose in milligrams (dose equivalent to an area under the curve [AUC] of 6) on day 1 of the cycle, for six cycles; patients who were assigned to the conventional regimen received paclitaxel at a dose of 175 mg per square meter on day 1 of a 21-day cycle, plus carboplatin (AUC, 6) on day 1 of the cycle, for six cycles. Patients in either group could opt to receive bevacizumab, starting from cycle 2, at a dose of 15 mg per kilogram of body weight, every 3 weeks until disease progression occurred.