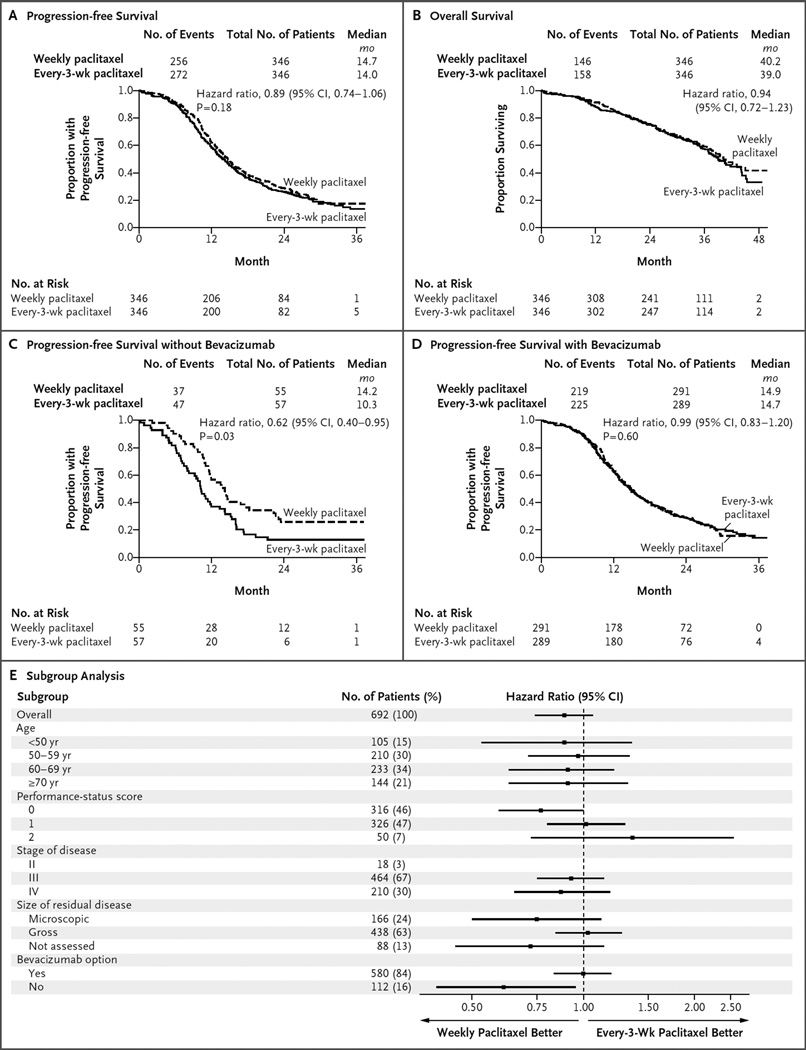
Figure 2. Primary and Subgroup Analyses of Progression-free Survival, According to Treatment Group.
In the overall intention-to-treat analysis, dose-dense weekly therapy with paclitaxel did not prolong progression-free survival, as compared with paclitaxel administered every 3 weeks (14.7 months and 14.0 months, respectively) (Panel A). In the case of progression-free survival, the hazard ratio is for disease progression or death. In the overall intention-to-treat analysis, weekly paclitaxel did not prolong overall survival, as compared with paclitaxel administered every 3 weeks (Panel B). In the case of overall survival, the hazard ratio is for death. No P value is available for the analysis of overall survival because the prespecified number of events has not occurred yet for this analysis. In the analysis of progression-free survival among patients who opted not to receive bevacizumab, weekly paclitaxel was associated with progression-free survival that was 3.9 months longer than that observed with paclitaxel administered every 3 weeks (14.2 vs. 10.3 months; hazard ratio for disease progression or death, 0.62; 95% CI, 0.40 to 0.95; P = 0.03) (Panel C). In the analysis of progression-free survival among patients who opted to receive bevacizumab, weekly paclitaxel did not prolong progression-free survival, as compared with paclitaxel administered every 3 weeks (14.9 months and 14.7 months, respectively; hazard ratio, 0.99; 95% CI, 0.83 to 1.20; P = 0.60) (Panel D). A forest plot of progression-free survival, according to randomized treatment, in subgroups defined according to prognostic factors is also shown (Panel E). Performance-status scores ranged from 0 (fully active) to 2 (ambulatory and capable of self-care but unable to work; up and about >50% of waking hours). In the subgroup of patients with stage II disease, there were too few patients to reliably estimate the treatment hazard ratio. The size of residual disease was not assessed in patients who underwent neoadjuvant therapy.