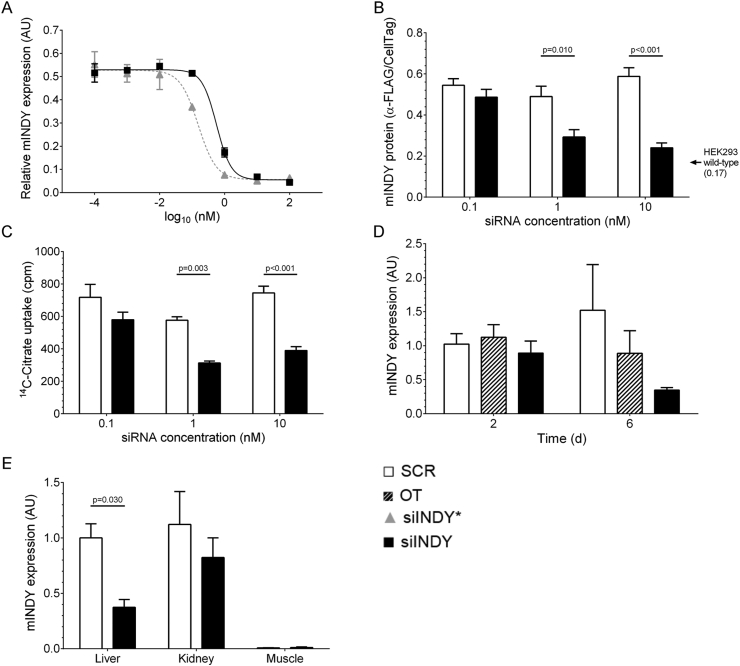
Figure 1.
In vitro (A–C) and in vivo (D and E) validation of mINDY-specific siRNA (siINDY). (A) Dose–response curves upon transfection of HEK293 cells stably overexpressing mINDY carrying a FLAG-tag (HEK293-mINDY) with siINDY and its unmodified form (siINDY*) (n = 2). (B) Transfection of HEK293-mINDY with siINDY reduced mINDY protein in a concentration-dependent manner, determined by anti-FLAG In-Cell Western Assay (n = 3). The black arrow indicates background fluorescence signal of HEK293 wild-type cells analyzed in parallel. (C) Transfection of HEK293-mINDY cells with siINDY reduced cellular citrate uptake (n = 3). (D) Pilot mouse study to evaluate the siRNA efficacy. mINDY mRNA expression in liver 2 and 6 days after injection normalized to GAPDH and control siRNA at day 2 (n = 3). (E) mINDY mRNA expression in liver, kidney, and muscle tissue after 8 weeks of siRNA treatment normalized to GAPDH and control siRNA in liver (n = 4). Data are represented as mean ± SEM. Two-way ANOVA with Bonferroni's multiple comparisons test. Scrambled control siRNA (SCR): open bars; off-target-specific mINDY-unrelated siRNA (OT): fasciated bars; mINDY-specific unmodified siRNA (siINDY*): grey triangle; mINDY-specific modified siRNA (siINDY): black bars.