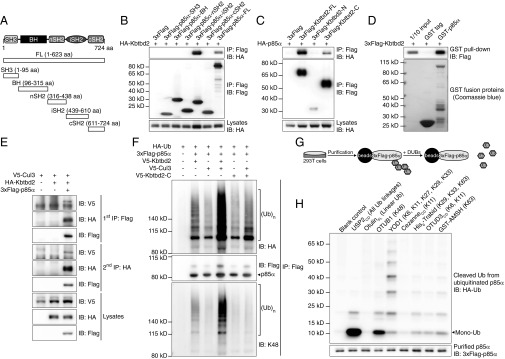
Fig. 5.
Interaction of KBTBD2 with Cul3 and p85α results in p85α ubiquitination. (A) Protein domains of mouse p85α and truncated forms for mapping the protein interaction region. (B) Immunoblot analysis of immunoprecipitates (Top and Middle) or lysates (Bottom) of 293T cells expressing HA-tagged KBTBD2 and 3×FLAG-tagged full-length (FL) or truncated p85α. (C) Immunoblot analysis of immunoprecipitates (Top and Middle) or lysates (Bottom) of 293T cells expressing HA-tagged p85α and 3×FLAG-tagged full-length (FL) or truncated KBTBD2. (D) Purified 3×FLAG-tagged KBTBD2 was incubated with GST or GST-tagged p85α. After GST pull-down, bound protein was analyzed by FLAG immunoblot (Top). The amounts of GST and GST-p85α were visualized by Coomassie blue staining (Bottom). (E) Immunoblot analysis of lysates of 293T cells expressing the indicated proteins and subjected to two-step IP to detect the three-protein complex. (F) IP analysis of lysates of 293T cells expressing HA-tagged Ub and the indicated proteins. (G) Flowchart of experimental procedures for data in H. HA-tagged Ub and 3×FLAG-tagged p85α were expressed in 293T cells. Immunoprecipitated 3×FLAG-p85α was treated with the indicated deubiquitinating enzymes (DUBs), which specifically cleave particular Ub linkages. The released HA-Ub in the reaction supernatant was immunoblotted with HA antibody. The deubiquitinated 3×FLAG-p85α was immunoblotted with FLAG antibody. (H) Immunoblot analysis of HA-tagged Ub (Top) released by the indicated DUBs from ubiquitinated 3×FLAG-tagged p85α (Bottom). Mono-Ub, monoubiquitin.