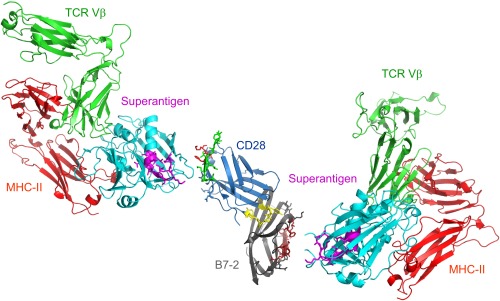
Fig. 8.
Schematic model for superantigen action, requiring direct binding of superantigen to costimulatory receptors B7-2 and CD28. Two superantigen molecules (cyan) (SEC3, a close relative of SEB) in complex with TCR Vβ (green) and MHC-II (red) extracellular domains (1jck.pdb, wherein SEB/MHC-II structure is superimposed on SEC3/TCR Vβ structure) (30) engage, through their freely accessible β-strand/hinge/α-helix domain (magenta) (12), the homodimer interface of B7-2 on the right (dimer interface residues within pB2-4, pB2-6, and pB2-7 are in brown) and the homodimer interface of CD28 on the left, respectively. Because the CD28/B7-2 complex structure remains unresolved, CD28 (1YJD.pdb) (15) was superimposed on CTLA-4 in the CTLA-4/B7-2 complex (1I85.pdb) (Fig. 4A); green and red CD28 dimer interface residues correspond to p2TA and p1TA (only HVK was resolved), respectively. Extracellular domains of CD28 and TCR are oriented such that they enter the T cell at the top, and those of the B7-2 and MHC-II molecule are oriented such that they enter the antigen-presenting cell at the bottom.