Abstract
Adenosquamous carcinoma (AdSC) is considered a rare variant of squamous cell carcinoma (SCC) which is considered to be more clinically aggressive. Data is very limited with very little case matched data on outcomes in the literature. It is also unknown whether the quantity of the adenocarcinoma component affects outcomes. A retrospective case–control study with 23 cases of AdSC and 1137 SCC controls was conducted. Cases were matched by anatomic subsite, treatment, and, for oropharynx, by p16 status. The following variables were adjusted for in the analysis: T classification (T1/T2 vs. T3/T4), N classification (N0–N2a vs. N2b–N3), age, and smoking. The adenocarcinoma component was quantified by the number of high power fields containing glands as low, moderate, or high. AdSCs had a significantly greater risk of disease recurrence but largely, the differences were not statistically significant. The quantity of adenocarcinoma did not correlate with disease recurrence or survival. This case–control study on AdSC shows modestly more clinically aggressive behavior than conventional SCC, even while controlling for p16 status for oropharyngeal cases. Further, it suggests the current definition of AdSC, with no minimum requirement for gland formation, is clinically accurate.
Keywords: Adenosquamous carcinoma, Squamous cell carcinoma, Head and neck, Survival, Case control
Introduction
Adenosquamous carcinoma (AdSC) is a rare variant of squamous cell carcinoma (SCC) that occurs in various organ systems and anatomic sites. In the head and neck, AdSC was first defined in 1968 by Gerughty et al. [1] in a series of 10 patients. They suggested that it is an aggressive tumor type. However, it was not until 1984 [2, 3] that it was separated from mucoepidermoid carcinoma (MEC) due to its morphologic differences and apparent poorer outcomes, even compared with high-grade MEC.
AdSCs were initially believed to originate from multipotent cells from the excretory ducts of minor salivary glands [1]. However, based on the finding of the expression patterns of a panel of different markers, particularly low-molecular-weight cytokeratins, it was suggested that oral AdSCs arise, from superficial squamous epithelium [4, 5]. Histologically, the World Health Organization (WHO) [6] defines AdSC as a combination of two components: true adenocarcinoma and SCC. The two components can occur in close proximity or may be relatively distinct and separated from each other, although the former is much more common. The SCC component can present either as in situ or invasive, and can be well, moderately, or poorly differentiated. Intercellular bridges and keratin pearls are usually easily identified, particularly on resection specimens. The adenocarcinoma element is typically found in the deeper parts of the lesion, and the gland formation should consist of “punched out” spaces with rounded contours and smooth edges. The latter feature helps to distinguish them from SCC with gland-like areas from acantholysis or degeneration, which typically form irregular spaces. Mucin production, intracytoplasmic or within the gland spaces, is typically present, but is not required for the diagnosis [6]. There is no minimum quantity of gland formation for the diagnosis. It may be challenging at times to distinguish AdSCs from MEC, which also displays a dual histomorphology, particularly in small tissue biopsy specimens. However, identification of areas of surface squamous dysplasia and/or frank squamous differentiation such as keratin pearls and/or intercellular bridges in AdSCs differentiates between the two.
AdSC appears to have the same risk factors as for conventional head and neck SCC. Oropharyngeal SCC caused by high-risk human papillomavirus (HPV) is now a well-recognized tumor entity whose incidence is on the rise [7–10]. However, there is little specific data about the relationship between HPV and AdSC. Masand et al. [11] studied head and neck AdSC for transcriptionally-active HPV and for the surrogate marker, p16, and found a few HPV-related oropharyngeal cases. These two patients had favorable outcomes compared to their HPV negative counterparts, neither developing progressive disease after treatment, suggesting that HPV-related AdSC of the oropharynx may have the same favorable prognosis as other SCC types.
The general consensus has been that AdSC has an aggressive clinical behavior with many patients presenting with high-stage tumors. Metastases to cervical lymph nodes are common, and locoregional and distant recurrences after treatment also frequently occur [11–16]. However, almost all of the data in the literature is in the form of small, retrospective studies. Further, it is unclear whether the aggressive behavior of these lesions in the head and neck is due to the inherent nature of the AdSC or is related to the anatomic subsites where they tend to occur (predominantly larynx and oral cavity), disease stage at presentation, or other associations. This necessitates case control studies between AdSC and conventional SCC in order to tightly control for variables other than histology. Only one such study has been published in the literature [15], and it, interestingly, did not find any significant differences in survival for AdSC relative to conventional SCC controls.
We performed a case control study of AdSC from a single institution using a large database of hospital cancer registry patients as controls. Also, since, unlike other organ systems such as lung and pancreas, the current WHO definition of head and neck AdSC indicates no minimum cutoff for the amount of SCC or adenocarcinoma components for the diagnosis [6], we analyzed outcomes by the amount of gland formation.
Materials and Methods
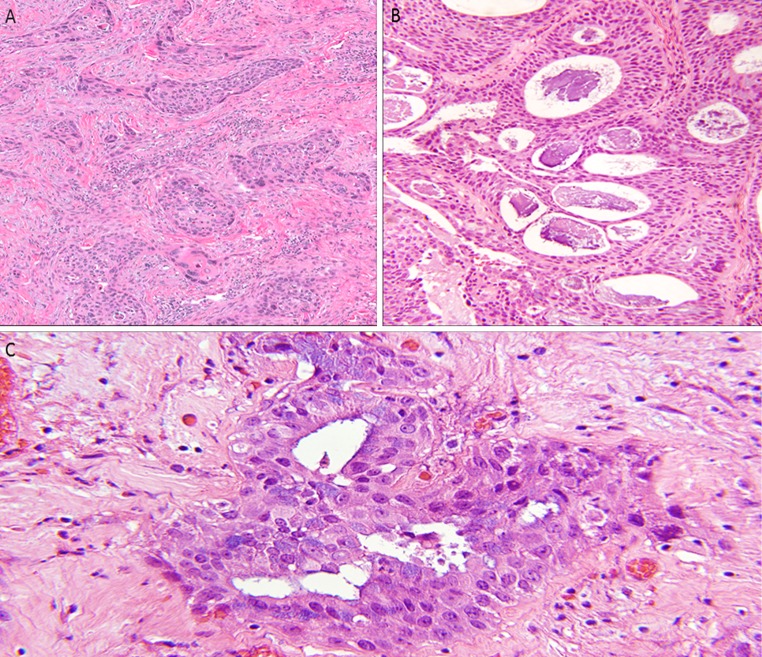
After approval by the Human Research Protection Office, the surgical pathology database of the Washington University in St. Louis/Barnes-Jewish Hospital Department of Pathology and Immunology was searched for all cases diagnosed as “adenosquamous carcinoma” with no other sub-specifications as to site or terminology. All cases from the head and neck region were identified. The entire pathology database from 1998 forward (to 2012) was queried, as the former was the year in which Copath was utilized as the information system. Two study pathologists (MM and JSL) reviewed all cases without knowledge of outcomes or other clinical features. The criteria used for inclusion of cases were those of the 2005 WHO Classification of head and neck tumors [6]. Specifically, the tumors had to show definitive squamous differentiation as well as areas of well-defined, smooth-edged gland formation. Mucin production, intracytoplasmic or intraluminal, was not a requirement for diagnosis, although all cases showed this histologically (Fig. 1). Special stains for mucin were performed in many cases as part of the routine clinical evaluation, but mucin stains were not utilized otherwise in this study for either review or inclusion/exclusion.
Fig. 1.
Adenosquamous carcinoma. a Conventional squamous cell carcinoma component (H&E ×100), b punched out gland spaces with associated mucin production in a more subtle background of squamous cell carcinoma (H&E ×200), c Tumor cells with intracytoplasmic mucin (H&E ×600)
Acantholytic SCC was excluded by identifying their pseudoglandular/alveolar areas where there was central acantholysis and cellular debris. The spaces in these tumors were irregular, and lacked rounded, “punched out” glands. AdSC was distinguished from mucoepidermoid carcinoma (MEC) by the presence of one or more of the following features of squamous differentiation including, intercellular bridges, keratin pearl formation, dyskeratosis, and SCC in situ of the overlying mucosa. AdSC also lacked the cystic change within tumor nests, a feature frequently seen in MEC [11]. The adenocarcinoma component was quantified by high power field (HPF) as low (≤10 HPF), moderate (11–49 HPF) or high (≥50 HPF) by a single study pathologist (MM).
Margin status on the AdSC patients was evaluated in the standard manner for the institution for all tumors. Surgeons provided defect specimens after the main tumor was removed for intraoperative pathology evaluation by frozen section. Additional tissue was resected when necessary. The margins were assessed by the pathologist on permanent section on the main specimen, and the findings combined with the results of the separate defect margin pieces to generate final margin status.
Immunohistochemistry was performed on the oropharyngeal AdSC and SCC cases using a Ventana Benchmark automated stainer with a p16 antibody (MTM Laboratories; clone E6H4; monoclonal; 1:1 dilution). The positive control was a known HPV mRNA (and p16) positive oropharyngeal SCC. Tumors were considered p16 positive if they had >75 % nuclear and cytoplasmic positivity.
Clinical follow-up data was obtained by chart review from the Departments of Radiation Oncology and Otolaryngology Head and Neck Surgery at Washington University. The Social Security Death Index was searched for those patients who had been lost to follow-up, to determine the date of death, if applicable.
The control group was drawn from an initial total of 3089 patients with larynx, hypopharynx, sinonasal, or oral cavity conventional SCC from Barnes-Jewish Hospital Oncology Data Services, and 312 patients with oropharyngeal SCC from an internal Department of Pathology and Immunology research database, between 2000 and 2009. Cases were then matched by site, treatment, and, for oropharynx cases, by p16 status. The following variables were not matched, but were adjusted for in the analysis: T stage (binary as T1/T2 vs. T3/T4), N stage (binary as N0–N2a vs. N2b–N3), age at diagnosis (the hazard of an event is calculated per 5 year increase), and smoking (binary as ever or former versus never). The number of controls per case was allowed to vary, and these ranged for each AdSC patient from 4 to 71 matched controls.
Overall survival (OS) was defined as the time from date of commencement of the treatment (either surgical resection or beginning of radiation or chemotherapy) to the date of last follow-up date or of death. Disease-specific survival (DSS) was calculated as the time from date of commencement of treatment to date of death in patients with known persistent or recurrent tumor at that time. Disease-free survival (DFS) was defined as the time from commencement of the treatment to the date of death due to any cause or to the date of first disease recurrence.
All patients’ tumors were primary and were those treated with curative intent. Cases that were identified as AdSC only in recurrent or metastatic tumors were excluded. In addition, all patients had to have at least 12 months of clinical follow-up. Two-year outcomes in the study group and controls were estimated by covariate-adjusted Cox proportional hazards models stratified by the 23 sets of cases and controls. Fisher’s exact test was utilized to calculate the association between quantitative categories of the adenocarcinoma component and 12-month disease recurrence status, where the recurrence endpoint is a proportion at 12 months and not time to recurrence. p values less than or equal to 0.05 were considered statistically significant. Hazard ratios and simple effect sizes were calculated as well, where appropriate.
Results
A total of 30 patients with AdSC were identified, 23 of whom met criteria for sufficient clinical follow up. Demographic and pathologic features are presented in Table 1. Most of the patients were men. The mean age was 60.4 years old, and most were smokers. The average tumor size was 2.8 cm (range 0.5–6.5 cm). Tumors originated primarily in the larynx, followed by the oral cavity and oropharynx. Among the oropharyngeal cases, two (2/5; 40 %) were positive for p16 immunohistochemistry, a surrogate marker for transcriptionally-active HPV and an established prognostic marker in oropharyngeal SCC and variants. The majority of patients had evidence of lymph node metastasis at the time of presentation and were diagnosed with Stage IV disease. Surgical approach was the most common method of treatment in our series, supplemented in nine patients with postoperative chemoradiation. All but two surgically treated AdSC patients had negative margins at the time of resection. The margin status on all cases was assessed by a pathologist intraoperatively with frozen sections and was further corrected if these were positive, by resection of additional tissue. One patient with a nasal cavity tumor had positive margins, and one with a larynx cancer had indeterminate margins because the tumor was present at the margins on the main specimen, and it wasn’t clear if the additional separate frozen section margins covered this area. This patient with indeterminate margin status, however, was disease free and alive after 54 months of follow-up, suggesting the original margin was most likely negative. The 1/18 (5.5 %) positive margin status rate for AdSC patients was slightly less than the 142/916 (15.5 %) rate for the matched conventional SCC control patients that had undergone surgery.
Table 1.
Clinical and pathologic characteristics of the study cohort (23 patients)
| Characteristics | n (%) |
|---|---|
| Male | 20 (86.9) |
| Female | 3 (13.1) |
| Mean age (years) | 60 |
| Positive smoking history | 17 (73.9) |
| Mean tumor size (cm) | 2.8 |
| AJCC Stage | |
| I | 3 (13.0) |
| II | 4 (17.3) |
| III | 1 (4.3) |
| IV | 15 (65.2) |
| Surgical treatment | 18 (78.2) |
| Non-surgical treatment | 5 (21.7) |
| Anatomic subsite | |
| Nasal cavity/paranasal sinuses | 2 (8.6) |
| Oral cavity | 5 (21.7) |
| Oropharynx | 5 (21.7) |
| 16 positive | 2 (40) |
| Larynx | 11 (47.8) |
| Lymphvascular space invasion | 9 (39.1) |
| Perineural invasion | 9 (39.1) |
| Lymph node metastases | 14 (60.8) |
| Local recurrence | 4 (17.3) |
| Regional recurrence | 4 (17.3) |
| Distant metastasis | 8 (34.7) |
AJCC American Joint Committee on Cancer
Follow-up data was available for all patients with an average duration of 38.3 months. Lung was the most common site for distant metastasis (seven of the eight patients with distant spread). During the follow-up period, nine patients (9/23; 39.1 %) died of their disease and three patients died of unrelated causes. Nine patients were alive with no evidence of disease (9/23; 39.1 %), and two patients were alive with disease.
After matching from the larger control patient databases to the AdSC patients, the final control group consisted of 1137 patients. T and N classifications were divided binarily and adjusted for as T1/T2 versus T3/T4 and N0–N2a versus N2b–N3, respectively. Similarly, age at diagnosis and smoking status were not matched but were adjusted for in the analysis. The number of controls per case was allowed to vary, and these ranged for each AdSC patient from one to 49 matched controls.
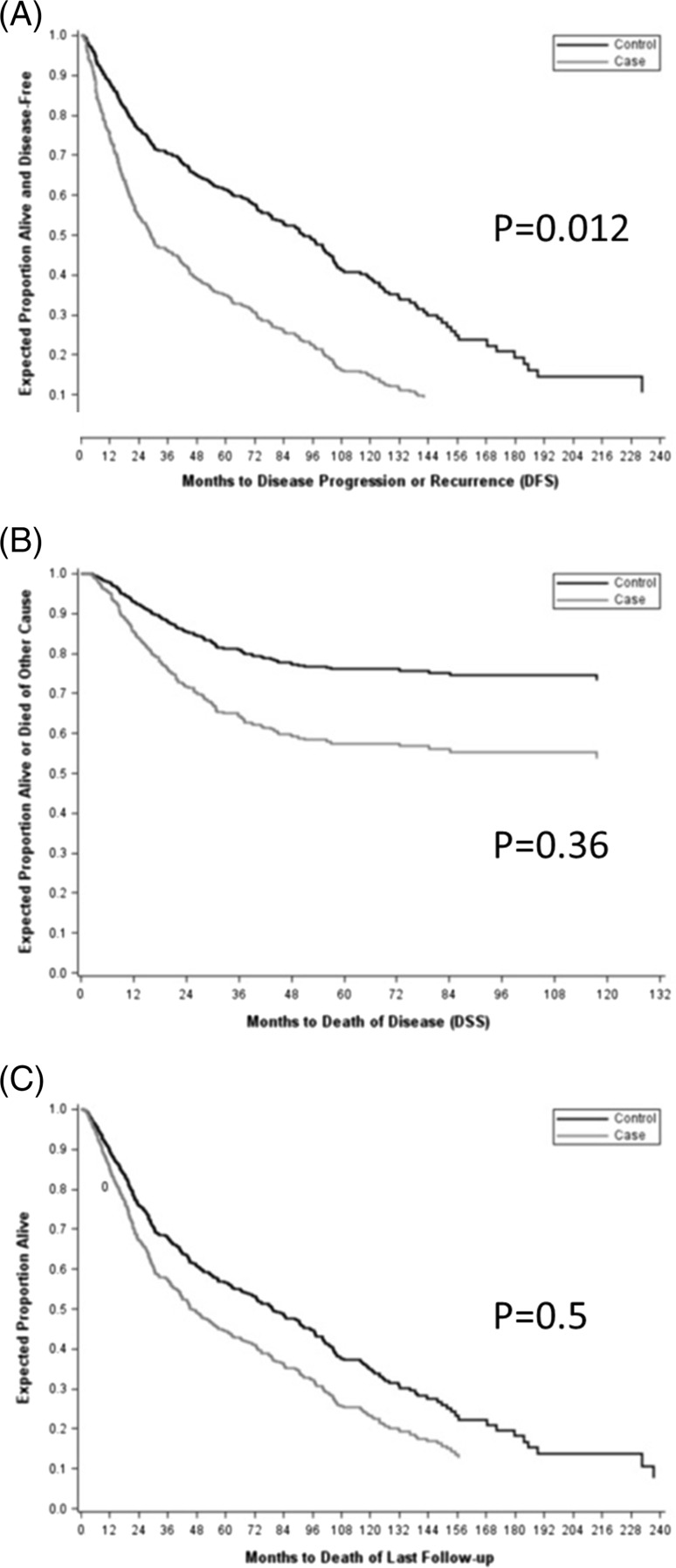
Median clinical follow up for the AdSC patients was 30.8 months and for the matched controls was 34.5 months. The 2-year OS, DFS, and DSS rates for the control group were 75 % [95 % CI (confidence interval) 72–77 %], 78 % (95 % CI 75–81 %), and 84 % (95 % CI 81–87 %) respectively. In the study group of AdSC patients, the 2-year OS, DFS and DSS rates were only 50 % (95 % CI 42–80 %), 54 % (95 % CI 32–73 %), 80 % (95 % CI 47–86 %), respectively.
By Cox proportional hazards models, the hazard of death or recurrence was 2.21 times for cases than for controls (p = 0.012, HR 2.21, 95 % CI 1.19, 4.11). DSS models, based on a smaller number of events (deaths of disease only), did not significantly distinguish the hazards of cases and controls (p = 0.36, HR 1.49, 95 % CI 0.638, 3.48). Overall survival, which includes all deaths but not recurrences, also was not demonstrably different among cases and controls (p = 0.50, HR 1.26, 95 % CI 0.642, 2.47) (Table 2) (Fig. 2).
Table 2.
Summary results of Cox proportional hazard model of disease free and specific survival (DFS and DSS) from matching by site, stage, p16 status, and treatment using model-based adjustment for T and N stage, age and smoking status and allowing the number of controls per case to vary
| Parameter | DFS | DSS | ||||
|---|---|---|---|---|---|---|
| p value | Hazard ratio | 95 % Confidence interval for HR | p value | Hazard ratio | 95 % Confidence interval for HR | |
| Case versus Control | 0.012 | 2.21 | (1.19, 4.11) | 0.36 | 1.49 | (0.638, 3.48) |
| Age at diagnosis (per 5 year increase) | <.0001 | 1.17 | (1.10, 1.24) | 0.025 | 1.10 | (1.01, 1.19) |
| Smoker versus Nonsmoker | 0.0534 | 1.44 | (0.995, 2.08) | 0.55 | 0.868 | (0.546, 1.38) |
| T Stage (T3–T4 versus T1–T2) | 0.0025 | 1.57 | (1.17, 2.10) | 0.0002 | 2.01 | (1.40, 2.90) |
| N Stage (N2b–N3 versus N0–N2a) | 0.0892 | 1.31 | (0.959, 1.79) | 0.0014 | 1.90 | (1.28, 2.82) |
DFS Disease free survival, DSS Disease specific survival, HR Hazard ratio, values in bold are statistically significant
Fig. 2.
Survival curves for adenosquamous carcinoma patients versus matched squamous cell carcinoma controls: a Disease free survival, b Disease specific survival, c Overall survival
The DFS and OS models indicate that the hazard of death or progression and the hazard of death of any cause increases by about 17 % per 5 year increase in age. In the DSS model, the hazard of death of disease increases by about 10 % per 5 year increase in age. In none of the DFS, DSS and OS models was smoking status (as assessed) demonstrably associated with the hazard of death or progression, death of disease, or death of any cause. Higher T classification (T3/T4 vs. T1/T2), was associated with a 57 % increase in the hazard of death or progression, a doubling of the hazard of death of disease, and a 76 % increase in the hazard of death of any cause. Higher N classification (N2b–N3 vs. N0–N2a) was associated with a 31 % increase in the hazard of death or progression, a 90 % increase in the hazard of death of disease and a 52 % increase in the hazard of death of any cause.
Of the 21 cases with all slides available for histologic review, 10 had high amounts (≥50 HPF) of the adenocarcinoma component, 6 had moderate amounts (11–49 HPF) and 5 had low amounts (≤10 HPF) (Table 3). The quantity of adenocarcinoma did not correlate with simple binary disease recurrence by Fisher’s exact test (p value = 0.14), nor with DFS of 1 year or more (p value = 0.14). It also did not correlate with DFS by log-rank test (p value = 0.11) (Table 3).
Table 3.
Adenocarcinoma component (quantified by high power field) and disease recurrence rates
| Adenocarcinoma component | DFS | n (%) | p value by log-rank test | DFS p value by Fisher’s exact test | |
|---|---|---|---|---|---|
| <12 months (%) | >12 months (%) | ||||
| ≤10 | 4 (80.0) | 1 (20.0) | 5 (23.8) | 0.11 | 0.14 |
| 11–49 | 1 (16.6) | 5 (83.3) | 6 (28.5) | ||
| ≥50 | 4 (40.0) | 6 (60.0) | 10 (47.6) | ||
DFS disease free survival
Discussion
AdSC of the head and neck has been described as a distinct head and neck tumor type for several decades, yet the clinical behavior of such tumors, particularly relative to conventional SCC, has been a topic of significant debate and relatively little scholarly investigation. Similar to other variants of head and neck SCC, the literature shows that AdSCs more commonly occur in men with a mean age at diagnosis of ~60 years [1, 11, 13]. The larynx is the most common subsite, followed by oral cavity and oropharynx. These generalities are supported by the results of the current study (Table 1). Regarding the clinical behavior, the general consensus has been, based on a small number of retrospective studies and literature reviews, that AdSCs have an aggressive behavior, with locoregional recurrence and death from disease being relatively common [1, 4, 11, 17]. However, until recently, there were no case control studies. Kass et al. [15] recently published a case control study of 42 head and neck AdSC and found no differences in clinical outcomes relative to conventional SCC (matched 1:2). Interestingly, they found a trend towards better survival in the AdSC patients. The series was enriched with oropharyngeal cases, however (10 cases or 24 % of all patients) in which they did not control for HPV/p16 status. Further, it included two nasopharyngeal cases without noting EBV status, and it also included a few tumors of the skin. These appear to be important limitations to their analysis as these sites of tumor can have viral associations which are well established good prognostic factors, and further because skin carcinomas are often small and superficial, frequently having very favorable prognosis.
In the current study, 34.7 % of AdSC patients developed locoregional recurrence, and 24.0 % experienced distant metastasis, most commonly to the lung. At 2 years of follow up, 45 % of the AdSC patients had died or had progression/recurrence of their disease, a rate more than double the patients in the control group. The poor outcome in AdSC in comparison with conventional SCC was further confirmed when factors generally known to influence survival were matched or controlled for, including anatomic subsite, T and N classification, treatment modality, smoking status, and age at diagnosis, and yet DFS still was significantly worse with a p value of 0.012 (Table 2). In considering the literature overall and the current case series, it appears to be the case that AdSC is clinically more aggressive than conventional SCC, but it may just be a matter of modest effect size. If the difference is only modest, then it will be difficult to demonstrate, particularly in underpowered, small studies. Margin sampling technique may also have an impact on locoregional recurrence, with higher rates of recurrence reported by some investigators when intraoperative margin sampling was performed from the tumor bed versus on the main resection specimen [18]. However, this most likely was not a contributing factor to the higher local aggressiveness in AdSCs, as all but two of the patients had negative margins and positive margin rates were even lower in the AdSC patients versus controls (5.5 vs. 15.5 %). This finding actually further supports the findings that AdSCs are clinically more aggressive compared to conventional SCCs. Because of this low frequency of positive/indeterminate margins, we chose not to include margin status in the matching.
It is not clearly understood why AdSCs may have aggressive behavior. Previous studies have suggested that predilection for perineural invasion may be a factor, which has been noted in 50 % of the cases in a series described by Keelawat [17] and in 40 % of the patients reported by Schick et al. [12]. Similarly, in our study, 39.1 % (9/23) of the tumors had evidence of perineural invasion. This is not significantly different than reported rates in the literature for conventional head and neck SCC, and, further, perineural invasion in general is not an overly powerful adverse prognostic factor in head and neck SCC.
In our series, most patients received surgery as their primary treatment modality (18/23; 78.2 %) with only five treated nonsurgically. Although studies regarding therapy in AdSC are limited and management strategies have not been standardized, surgery seems to be the treatment of choice, with likely added therapeutic advantage with adjuvant chemoradiation when it would be otherwise indicated [17].
It is well known that oropharyngeal SCC patients have a much better prognosis when their tumors are associated with transcriptionally-active HPV [10] and as a result, p16 immunohistochemistry has been used as a sensitive surrogate marker for high-risk HPV. Several histologic variants of SCC, including nonkeratinizing SCC, basaloid SCC, papillary SCC, and undifferentiated carcinoma [9, 19, 20], have been shown to harbor high risk HPV when arising in the oropharynx. In a recent study, Masand et al. [11] studied the relationship of head and neck AdSC to HPV in a series of 18 cases by utilizing high risk HPV RNA in situ hybridization (ISH) assay along with the p16 IHC and found only 3 cases harboring transcriptionally-active HPV and showing overexpression of p16, two of which were in the oropharynx and one in the nasal cavity, with a suggestion of a better clinical outcome in the oropharyngeal patients since neither developed disease recurrence. In the current study, only 2 of the 5 oropharyngeal cases were p16 positive by IHC. The two p16 positive patients had long term survival with no disease recurrence, but the numbers are simply too small to discern whether HPV/p16 provides the same prognostic benefit in oropharyngeal AdSC as it does for all other HPV-related SCC variants arising there.
We assessed whether the amount of glandular component affects behavior. By classifying the cases into three groups based on the number of high-power field (HPF) foci of glandular differentiation, we found no significant difference in survival between the three groups (Table 3). While our numbers are small and not statistically significant, interestingly, there actually seemed to be a trend toward better outcomes in patients with more gland formation in their tumors, not less. This is highlighted in Table 2, showing 60 % of group 3 patients having a disease-free survival of greater than 12 months as opposed to only 20 % of patients in group 1 surviving more beyond 12 months. Whatever the case, there is nothing here to suggest that the current WHO definition of AdSC, where no minimum amount of gland formation is specified, should be changed. For all of the issues explored in the current study, future studies with larger number of cases are needed to address them in a more statistically powerful manner.
In summary, this case–control study on survival in head and neck AdSC compared to conventional SCC shows that AdSC patients have higher disease recurrence rates and poorer survival, even when controlling for p16/HPV status in the oropharyngeal cases, but the effect size appears to be modest. It also suggests that the current WHO definition of AdSC, which does not require any minimum quantity of gland formation for the diagnosis, is a clinically accurate one. Given the accumulated evidence from this study and the prior literature, when considering treatment of patients with AdSC of the head and neck, it should probably be considered as being on the more clinically aggressive spectrum relative to conventional SCC with higher propensity for disease recurrence.
References
- 1.Gerughty RM, Hennigar GR, Brown FM. Adenosquamous carcinoma of the nasal, oral and laryngeal cavities. A clinicopathologic survey of ten cases. Cancer. 1968;22:1140–1155. doi: 10.1002/1097-0142(196811)22:6<1140::AID-CNCR2820220610>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Evans HL. Mucoepidermoid carcinoma of salivary glands: a study of 69 cases with special attention to histologic grading. Am J Clin Pathol. 1984;81:696–701. doi: 10.1093/ajcp/81.6.696. [DOI] [PubMed] [Google Scholar]
- 3.Accetta PA. Mucoepidermoid carcinoma–the real enemy among them. Am J Clin Pathol. 1984;82:512–513. doi: 10.1093/ajcp/82.4.512. [DOI] [PubMed] [Google Scholar]
- 4.Alos L, Castillo M, Nadal A, Caballero M, Mallofre C, Palacin A, et al. Adenosquamous carcinoma of the head and neck: criteria for diagnosis in a study of 12 cases. Histopathology. 2004;44:570–579. doi: 10.1111/j.1365-2559.2004.01881.x. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca FP, Ramos LMA, Vargas PA, de Almeida OP, Lopes MA, Santos-Silva AR. Oral adenosquamous carcinoma: evidence that it arises from the surface mucosal epithelium. Histopathology. 2012;61:321–323. doi: 10.1111/j.1365-2559.2012.04257.x. [DOI] [PubMed] [Google Scholar]
- 6.Cardesa A, Zidar N, Alos L. Adenosquamous carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidranksy D, editors. World Health Organization classification of tumours—pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 130–131. [Google Scholar]
- 7.Bishop JA, Lewis JS, Rocco JW, Faquin WC. HPV-related squamous cell carcinoma of the head and neck: An update on testing in routine pathology practice. Semin Diagn Pathol. 2015;32(5):344–351. doi: 10.1053/j.semdp.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Lewis JS, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehrad M, Carpenter DH, Chernock RD, Wang H, Ma X-J, Luo Y, et al. Papillary squamous cell carcinoma of the head and neck: clinicopathologic and molecular features with special reference to human papillomavirus. Am J Surg Pathol. 2013;37:1349–1356. doi: 10.1097/PAS.0b013e318290427d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masand RP, El-Mofty SK, Ma X-J, Luo Y, Flanagan JJ, Lewis JS. Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of the literature. Head Neck Pathol. 2011;5:108–116. doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schick U, Pusztaszeri M, Betz M, Ghadjar P, Demiroz C, Kaanders JHAM, et al. Adenosquamous carcinoma of the head and neck: report of 20 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:313–320. doi: 10.1016/j.oooo.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Napier SS, Gormely JS, Newlands C, Ramsay-Baggs P. Adenosquamous carcinoma. A rare neoplasm with an aggressive course. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:607–611. doi: 10.1016/S1079-2104(05)80103-9. [DOI] [PubMed] [Google Scholar]
- 14.Kothari RK, Ghosh A, Bhattacharyya SK, Ghosh SK. Adenosquamous carcinoma of oral cavity: a case report. J Indian Med Assoc. 2007;105:531–532. [PubMed] [Google Scholar]
- 15.Kass JI, Lee SC, Abberbock S, Seethala RR, Duvvuri U. Adenosquamous carcinoma of the head and neck: molecular analysis using CRTC-MAML FISH and survival comparison with paired conventional squamous cell carcinoma. Laryngoscope. 2015;125:E371–E376. doi: 10.1002/lary.25519. [DOI] [PubMed] [Google Scholar]
- 16.Dubal PM, Unsal AA, Echanique KA, Vazquez A, Reder LS, Baredes S, et al. Laryngeal adenosquamous carcinoma: a population-based perspective. Laryngoscope. 2016;126(4):858–863. doi: 10.1002/lary.25704. [DOI] [PubMed] [Google Scholar]
- 17.Keelawat S, Liu CZ, Roehm PC, Barnes L. Adenosquamous carcinoma of the upper aerodigestive tract: a clinicopathologic study of 12 cases and review of the literature. Am J Otolaryngol. 2002;23:160–168. doi: 10.1053/ajot.2002.123462. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell JH, Thompson LD, Brandwein-Gensler MS, Weiss BG, Canis M, Purgina B, Prabhu AV, Lai C, Shuai Y, Carroll WR, Morlandt A, Duvvuri U, Kim S, Johnson JT, Ferris RL, Seethala R, Chiosea SI. Early oral tongue squamous cell carcinoma: sampling of margins from tumor bed and worse local control. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1104–1110. doi: 10.1001/jamaoto.2015.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernock RD, Lewis JS, Zhang Q, El-Mofty SK. Human papillomavirus-positive basaloid squamous cell carcinomas of the upper aerodigestive tract: a distinct clinicopathologic and molecular subtype of basaloid squamous cell carcinoma. Hum Pathol. 2010;41:1016–1023. doi: 10.1016/j.humpath.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter DH, El-Mofty SK, Lewis JS. Undifferentiated carcinoma of the oropharynx: a human papillomavirus-associated tumor with a favorable prognosis. Mod Pathol. 2011;24:1306–1312. doi: 10.1038/modpathol.2011.87. [DOI] [PubMed] [Google Scholar]