Figure 4.
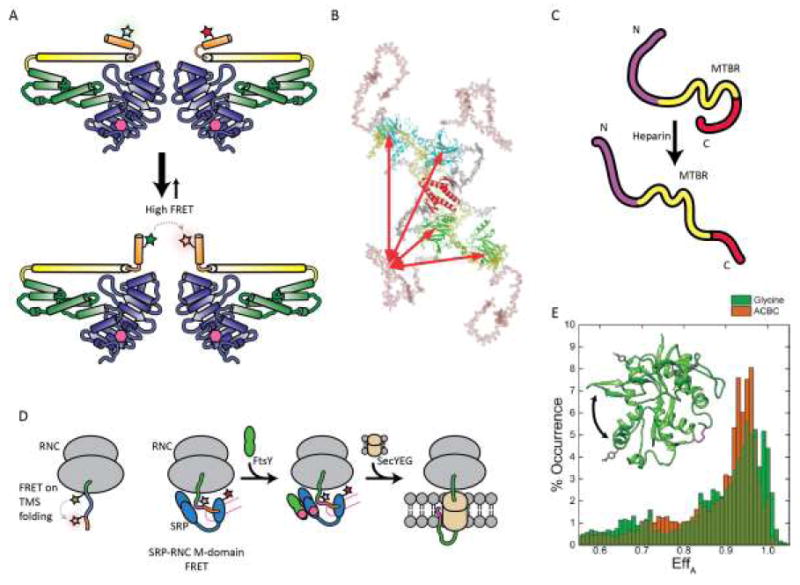
FRET based studies of protein folding and interactions. (A) FRET and double electron-electron resonance based observations of close spatial proximity between two α13 helices in the human glutamate binding protein 1 homodimer. (B) Schematic model of full length p53 conformations; DNA binding domain is shown in green or cyan, tetramerization domain shown in gray, N-terminal domain in salmon, and C-terminus in yellow. (C) Model for tau conformational changes in the presence of heparin based on smFRET and coarse-grained modeling. (D) FRET -pair systems for studying transmembrane sequence (TMS) folding in ribosomal nascent chain complex (RNC) and RNC-signal recognition particle (SRP) interaction during cotranslational translocation to membranes. (E) Comparison of smFRET histograms of NMDA glycine binding domain (GluNl) in the presence of full agonist glycine (green) or partial agonist 1 -amino-1-cyclobutane carboxylic acid (ACBC; orange). Inset: crystal structure of the GluNl agonist-binding domain showing AcF side chains (grey) and native Cys (magenta, disulfide; yellow, free Cys) in stick form; arrow indicates domain closure. (B) Reproduced from ref. [50] , Copyright 2009, National Academy of Sciences. (E) Adapted from ref. [59], Copyright 2015, the American Society for Biochemistry and Molecular Biology.
