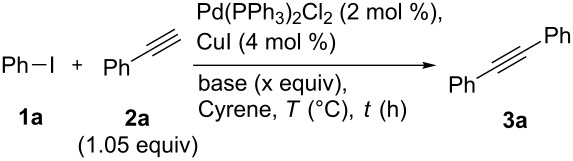
Table 1.
Reaction optimisation and comparison with existing solvents.a
 | ||
| Entry | Reaction conditions | 3a (%)b |
| 1 | 0.1 M, Et3N (3 equiv), 20 °C, 5 h | 94 |
| 2 | 0.3 M, Et3N (3 equiv), 20 °C, 5 h | 98 |
| 3 | 0.5 M, Et3N (3 equiv), 20 °C, 5 h | 100 |
| 4 | 0.5 M, K3PO4 (3 equiv), 20 °C, 5 h | –c |
| 5 | 0.5 M, Cs2CO3 (3 equiv), 20 °C, 5 h | –c |
| 7 | 0.5 M, Et3N (1.1 equiv), 20 °C, 5 h | 98 |
| 8 | 0.5 M, Et3N (1.1 equiv), 30 °C, 1 h | 96 |
| 9d | 0.5 M, Et3N (1.1 equiv), 30 °C, 1 h | 81 |
| 10e | 0.5 M, Et3N (1.1 equiv), 30 °C, 1 h | 87 |
a1 (1 equiv, 0.25 mmol), 2 (1.05 equiv, 0.26 mmol), Pd(PPh3)2Cl2 (2 mol %), CuI (4 mol %), base (see table), Cyrene, temperature (see table), time (see table), N2. bIsolated yield. cReaction mixture solidified, product was not isolated. dTHF used as solvent. eDMF used as solvent.
