Table 2.
DIBAL reductions of quinolin-2-ones 23a–e using the optimised method to synthesise 22.a
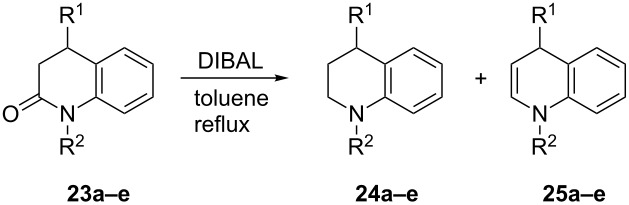 | |||||
| Quinolin-2-one | R1 | R2 | 23 (%) | 24 (%) | 25 (%) |
| 23a | Ph | H | 0 | 100 | 0 |
| 23b | Ph | Me | 0 | 38 | 62 |
| 23c | H | H | 12 | 88 | 0 |
| 23d | H | Et | 0b | 63b | 21b |
| 23e | H | Bn | 0c | 35c | Tracec |
aDIBAL (1.0 M in toluene, 1.2 equivalents or 2.0 equivalents for 23a and 23c) added to a refluxing solution of quinolin-2-one 23a–e (0.5–3.0 mmol) in toluene stirred rapidly at reflux until TLC analysis indicated completion. Then quenched with 20% aq w/v NaOH, extracted with EtOAc, washed with H2O and brine, dried (MgSO4) and evaporated. Yields of 23a–e, 24a–e and 25a–e were estimated from 1H NMR analysis of the crude mixture (see Supporting Information File 1). bA ring-opened ethylaniline (9%) and another byproduct were also present (7%). cA ring-opened benzylaniline was also present (61%).
