Table 3.
Comparing the reactivity of 6-iodo-THQ 21 and 6-bromo-THQ 26 in a typical Suzuki reaction with boronic ester 27 to give biaryl 28.
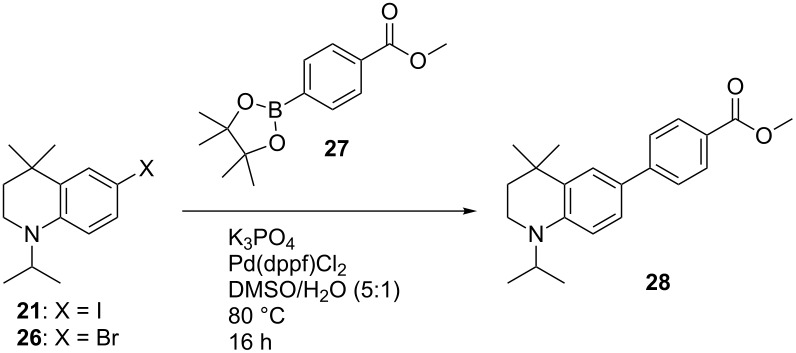 | |||
| THQ | Halide | Conversion of starting materiala | Isolated yield of 28 |
| 21 | I | 100% | 68% |
| 26 | Br | 56% | N/Ab |
aConversion of the starting THQs 21/26 to the coupling product 28 was estimated by comparing the integrals of the CH2 adjacent to the nitrogen (the 2-position) in the crude 1H NMR spectrum. bNot isolated.
