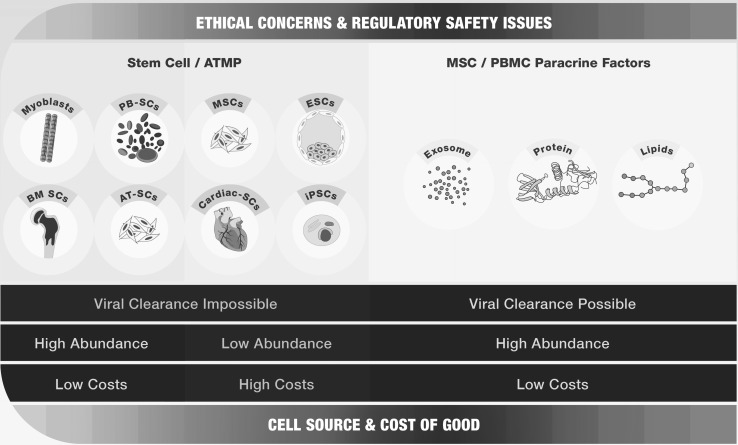
Fig. 4.
Ethical, economic, and safety considerations of stem cell-based and paracrine factor-based therapies. In contrast to stem cell-based therapies, paracrine factor-based therapies can be seen as an economic, ethical, and fully acceptable therapeutic strategy lacking significant safety issues and restrictions in production capacity. Viral clearance methods are not possible in stem cell-based therapies containing myoblasts, bone marrow stem cells (BM-SCs), peripheral blood stem cells (PB-SCs), adipose tissue-derived stem cells (AT-SCs), mesenchymal stem cells (MSCs), cardiac stem cells (Cardiac-SCs), embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs). In addition, ESC- and iPSC-based therapies are associated with high costs for cell manufacturing and low cell numbers. Stem cell-based therapies with ESCs, and especially iPSCs, also have ethical and safety concerns because these cells have on embryonic source or bear a risk of malignant transformation. In contrast, paracrine factors derived from PBMCs or MSCs, including exosomes, proteins, and lipids, can be subjected to viral clearance methods, guaranteeing a viral-free medical drug. In addition, paracrine factors, especially from peripheral blood mononuclear cells (PBMCs), can be produced and stored in high amounts with low costs