Figure 7.
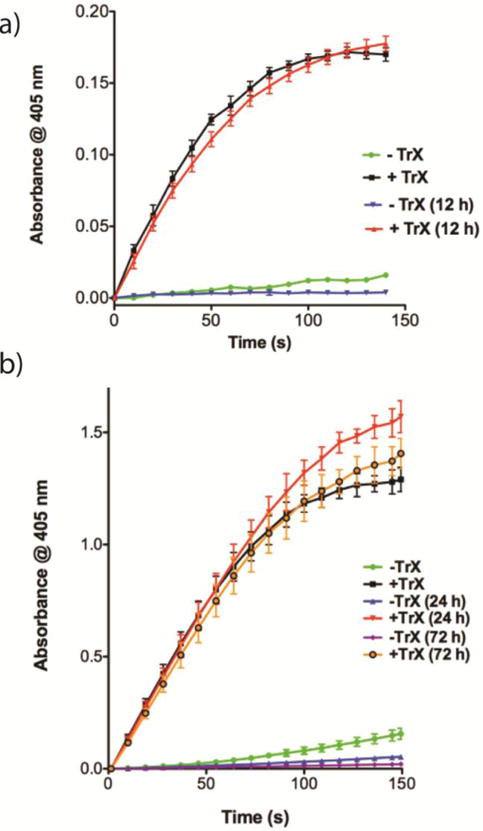
a) Centrifugation after sorafenib:CR (25:1, 500 μM, KPi) incubation with AmpC resulted in a red pellet that could be easily re-suspended in KPi buffer (n = 3, mean ± SD). Addition of β-lactamase substrate CENTA™ substrate led to limited substrate cleavage (green line). Colloid disruption using 0.1% TrX restored activity and resulted in substrate cleavage as indicated by the increase in absorbance at 405 nm (black line). Colloid-enzyme complexes were left for 12 h, after which nearly identical activity was found for solutions without (blue line) and with (red line) TrX addition. b) Studies with trypsin revealed similar trends as outlined above (n = 3, mean ± SD). Activity here was monitored after 0, 24 and 72 h. For both a) and b), activity of the released enzymes between 0 and 12, 24 or 72 h is not statistically significant.
