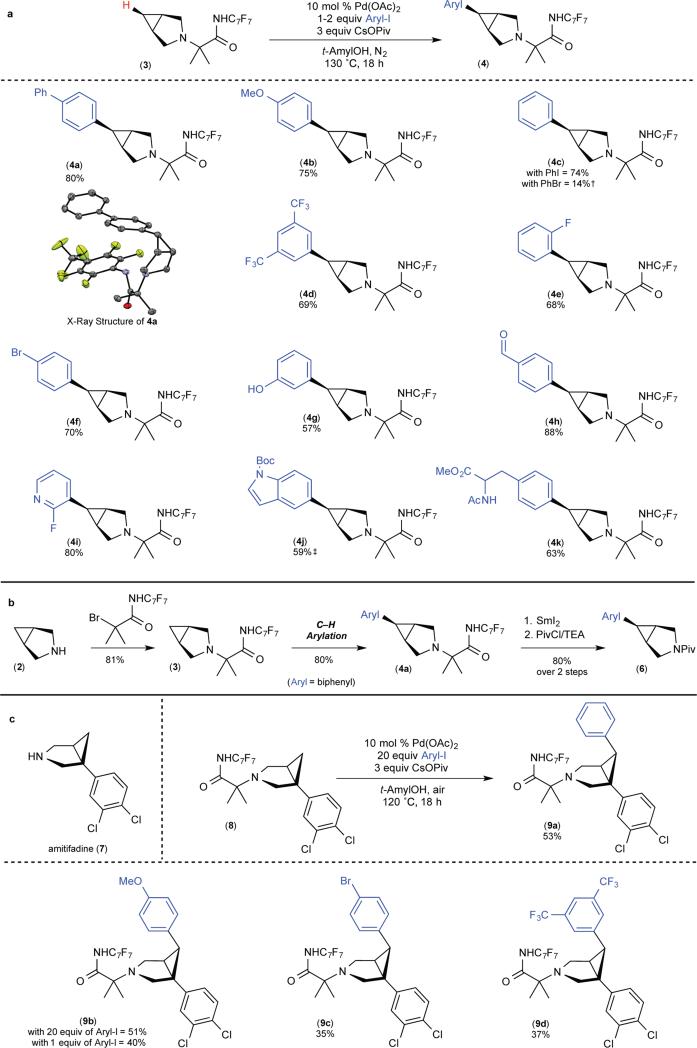
Figure 3. Transannular C–H arylation of 3-azabicyclo[3.1.0]hexane core.
a, Scope of C–H arylation with respect to the aryl iodide. b, Relevant steps in overall transformation: installation of directing group, C–H arylation and SmI2-mediated removal of directing group (Aryl = biphenyl). PivCl, pivaloyl chloride; TEA, triethylamine. c, C–H arylation applied to amitifiadine. All yields are reported for pure isolated material. †, Reaction was conducted using 20 equiv of PhBr; yield determined by GC. ‡, Reaction was conducted under modified conditions. See supplementary information for full details.