Figure 7. Viral transduction of human sensory neurons for expression of optogenetic tools to manipulate neuronal firing.
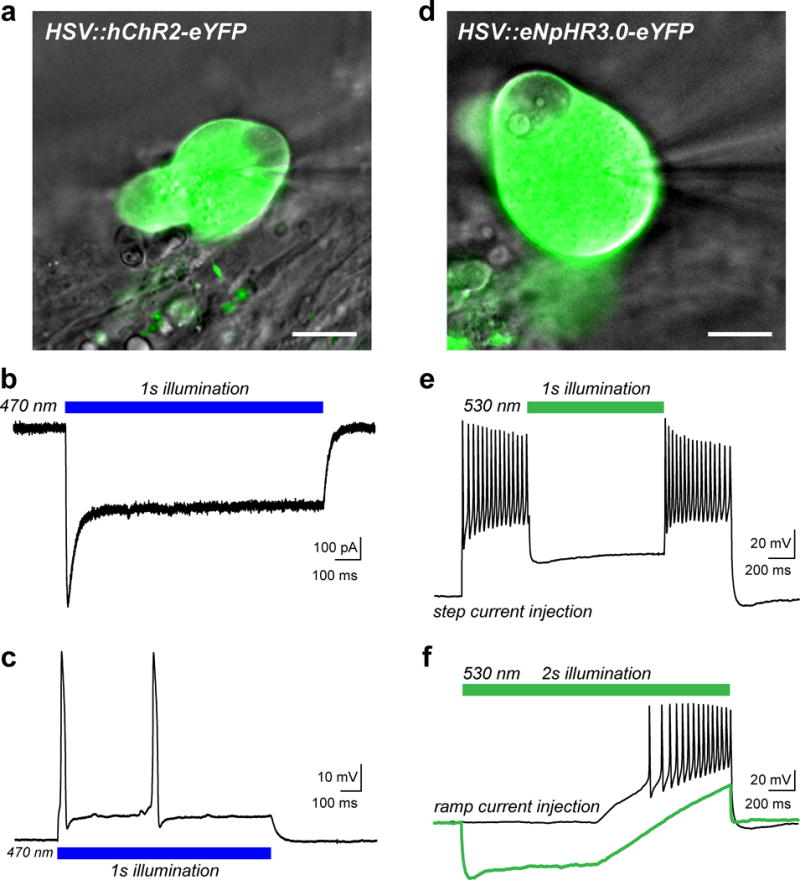
(a) Overlay of a DIC image of a human sensory neuron (gray) transduced with herpes simplex virus (HSV) carrying a transgene for the humanized excitatory opsin, channelrhodopsin-2 (hChR2-eYFP, green). Viruses were added at a concentration of 3.2×108 vector genomes/ml after 8 DIV and cells were examined 1–2 days later. The patch-pipette is visible on the right side of the image. (b) Representative inward photocurrent induced by illumination with blue LED light (blue bar, 10 mW/mm2) as previously described24. (c) Current-clamp recording showing action potential (AP) firing in response to blue light illumination as in (b). (d) DIC image (gray) of a sensory neuron that was transduced with HSV carrying the inhibitory Cl− pump halorhodopsin (eNpHR3.0-eYFP, green). (e) Voltage trace from a current-clamp recording in response to a 2s suprathreshold step current injection to elicit AP firing. Neuronal activity was acutely silenced by green LED illumination (green bar, 30 mW/mm2). (f) Current-clamp trace showing AP firing elicited by a ramp protocol of suprathreshold depolarizing current injection (black trace). Illumination with green LED light (30 mW/mm2) hyperpolarized the neuron and blocked AP discharge to the stimulus (green trace). Scale bars represent 20 μm. HSV vectors used include: CMV:ChR2(H134R)-eYFP and CMV:eNpHR3.0-eYFP, (both at 3.2×108 vector genomes/ml) from Rachael Neve at the MIT McGovern Viral Core Facility.
