Abstract
The zero-tannin trait in lentil is controlled by a single recessive gene (tan) that results in a phenotype characterized by green stems, white flowers, and thin, transparent, or translucent seed coats. Genes that result in zero-tannin characteristics are useful for studies of seed coat pigmentation and biochemical characters because they have altered pigmentation. In this study, one of the major groups of plant pigments, phenolic compounds, was compared among zero-tannin and normal phenotypes and genotypes of lentil. Biochemical data were obtained by liquid chromatography-mass spectrometry (LC-MS). Genomic sequencing was used to identify a candidate gene for the tan locus. Phenolic compound profiling revealed that myricetin, dihydromyricetin, flavan-3-ols, and proanthocyanidins are only detected in normal lentil phenotypes and not in zero-tannin types. The molecular analysis showed that the tan gene encodes a bHLH transcription factor, homologous to the A gene in pea. The results of this study suggest that tan as a bHLH transcription factor interacts with the regulatory genes in the biochemical pathway of phenolic compounds starting from flavonoid-3’,5’-hydroxylase (F3’5’H) and dihydroflavonol reductase (DFR).
Introduction
Phenolic compounds are characterized by the presence of at least one -OH group and an aromatic ring. They include phenolic acids, stilbenes, and flavonoids such as flavanones, flavones, dihydroflavonols, flavonols, flavan-3-ols, anthocyanidins, and proanthocyanidins [1]. Phenolics are associated with health benefits including antioxidant activity and protection against diseases such as cardiovascular disorders, cancer, HIV, and diabetes [2–6]. Physical removal of the seed coat of lentils leads to improved iron bioavailability [7], probably due to the removal of phenolic compounds and the implication that these compounds interfere with iron nutrition [8].
The phenylpropanoid pathway plays an important role in the biosynthesis of different groups of phenolic compounds [1]. The enzymes and related genes for branches of the pathway have been defined extensively in model plants [9–12]. Among the numerous phenotypic traits controlled by this pathway, pigmentation has been well characterized in several plant species. Generally, variability in black, purple, red, pink, brown, and yellow colouration in many tissues is the result of different combinations of the end products of this pathway [13]. A number of transcription factors (TFs) and modifying enzymes that influence gene expression in this pathway have been identified. The conserved TFs R2R3-MYB, WD-repeat (WDR), and basic-helix-loop-helix (bHLH) form an activation complex called the MYB-bHLH-WD (MBW) repeat complex [14] that controls the phenylpropanoid pathway in most plants.
Lentil (Lens culinaris Medikus) is an important grain legume crop that provides a good source of protein, carbohydrates, and micronutrients for humans. The primary seed coat colour in most market classes of lentil is determined by two independent genes: gray ground colour (Ggc) and tan ground colour (Tgc) [15]. The dominant and recessive allelic combinations of these two genes result in seed coats that are brown (Ggc Tgc), gray (Ggc tgc), tan (ggc Tgc), or green (ggc tgc) and characterize specific market classes. Phenolic compound profiling of some lentil market classes has been reported [16–21], but there is no information about the specific phenolic compounds and associated phenylpropanoid pathway genes related to the basic set of lentil seed coat colours.
The lentil market class known as ‘zero-tannin’ is determined by expression of a single recessive gene, tan [22, 23]. Homozygous recessive tan is epistatic to Tgc, but not to Ggc [15]. In tan genotypes, the expression of the dominant Ggc produces a gray translucent seed coat, while the recessive ggc results in a transparent seed coat. The colour of seed coats in tan genotypes does not change during storage [24] or cooking. The thinner seed coat results in faster cooking, easier dehulling, and a rounder seed shape. These characteristics are desirable for processors and consumers, creating opportunities for breeding lentils with higher value. Zero-tannin seeds also imbibe water more quickly leading to imbibitional injury at the time of germination [24, 25], a negative agronomic characteristic that can be overcome using modified techniques such as seed coating.
The tan gene also influences pigmentation of stems and flowers. Non-mutant lentil plants have reddish stems, purple veins on floral tissues, and thicker, pigmented seed coats [22]. The tan phenotype is characterized by green stems and white flowers. This set of traits is similar to Mendel’s A gene in pea (Pisum sativum) [26], which encodes a bHLH TF that has a regulatory function with pleiotropic effects [27]. The absence of pigmentation in pea is the result of a mutation in this bHLH with mis-spliced mRNA caused by a premature stop codon [27]. The striking similarities between the two sets of phenotypes suggest that the lentil homologue of the pea A gene could be the lentil tan gene.
The objective of this study was to compare the phenolic compound profiles obtained by liquid chromatography-mass spectrometry (LC-MS) of seed coats in the zero-tannin (tan) and normal (Tan) genotypes of lentil (Fig 1) along with the corresponding genotypic data. This information will help to further characterize tan as well as segments of the phenylpropanoid pathway that are influenced by this gene.
Fig 1. Images of lentil seeds with normal brown (Ggc Tgc Tan), zero-tannin gray (Ggc tan), and zero-tannin transparent (ggc tan) lentil seed coats.
Results
Biochemical Analysis
The retention time and the optimized molecular and fragment ions of the standards and the potential compounds from different sub-classes of phenolic compounds are reported in Tables 1 and 2. Among the analyzed compounds, 4-hydroxybenzoic acid, chlorogenic acid, and trans-ferulic acid (phenolic acids sub-class), resveratrol aglycone (stilbenes sub-class), naringenin and flavanone aglycones (flavanones sub-class), apigenin, flavone, and luteolin-3',7-di-O-glucoside (flavones sub-class), kaempferol-3-O-glucoside, kaempferol-7-O-neohesperidoside, quercetin, quercetin-3-O-glucoside, quercetin-3-O-galactoside, quercetin-4'-O-glucoside, quercetin-3,4'-di-O-glucoside (flavonols sub-class), epigallocatechin gallate and epicatechin gallate (flavan-3-ols sub-class), procyanidin A2 and B2 (proanthocyanidin sub-class), and the anthocyanidins were not detected in any of the tested lentil samples.
Table 1. Phenolic compound characteristics in selected reaction monitoring (SRM), including sub-class, retention time, molecular ion, and optimum fragment ion in positive or negative mode.
| Compound | Sub-class | Retention time (min) | Mode | Molecular ion (m/z) | Fragment ion (m/z) |
|---|---|---|---|---|---|
| Protocatechuic acid | Phenolic acids | 3.8 | Neg | 153 | 109 |
| Vanillic acid-4-β-D-glucoside | Phenolic acids | 4.2 | Neg | 329 | 167 |
| (−)-Gallocatechin | Flavan-3-ols | 4.7 | Neg | 305 | 125 |
| 4-Hydroxybenzoic acid | Phenolic acids | 5.0 | Neg | 137 | 93 |
| Procyanidin B1 | Proanthocyanidins | 5.9 | Neg | 577 | 289 |
| Catechin-3-glucoside* | Flavan-3-ols | 6.5 | Neg | 451 | 137 |
| (-)-Epigallocatechin | Flavan-3-ols | 6.5 | Neg | 305 | 125 |
| (+)-Catechin | Flavan-3-ols | 6.6 | Neg | 289 | 203 |
| ±-Catechin-2,3,4-13C3 | IS | 6.6 | Neg | 292 | 206 |
| Chlorogenic acid | Phenolic acids | 6.9 | Neg | 353 | 191 |
| Delphinidin-3-β-D-glucoside | Anthocyanidins | 6.9 | Neg | 463 | 300 |
| Procyanidin B2 | Proanthocyanidins | 7.1 | Neg | 577 | 289 |
| (-)-Epicatechin | Flavan-3-ols | 8.0 | Neg | 289 | 203 |
| Dihydromyricetin | Dihydroflavonols | 8.2 | Neg | 319 | 193 |
| Kaempferol dirutinoside* | Flavonols | 8.6 | Neg | 901 | 755 |
| Cyanidin-3-O-rhamnoside | Anthocyanidins | 8.7 | Neg | 431 | 285 |
| trans-p-Coumaric acid | Phenolic acids | 8.7 | Neg | 163 | 119 |
| Malvidin-3-O-galactoside | Anthocyanidins | 8.7 | Neg | 491 | 313 |
| (-)-Epigallocatechin gallate | Flavan-3-ols | 8.8 | Neg | 457 | 169 |
| Quercetin-3,4'-di-O-glucoside | Flavonols | 9.7 | Neg | 625 | 463 |
| trans-Ferulic acid | Phenolic acids | 9.9 | Neg | 193 | 134 |
| Dihydroquercetin | Dihydroflavonols | 10.2 | Neg | 303 | 125 |
| Kaempferol-3-O-robinoside-7-O-rhamnoside | Flavonols | 10.2 | Neg | 739 | 593 |
| Luteolin-3',7-di-O-glucoside | Flavones | 10.5 | Neg | 609 | 285 |
| Procyanidin A2 | Proanthocyanidins | 10.5 | Neg | 575 | 285 |
| Resveratrol-3-β-mono-D-glucoside | Stilbenes | 10.5 | Neg | 389 | 227 |
| (-)-Epicatechin gallate | Flavan-3-ols | 10.6 | Neg | 441 | 169 |
| Myricetin-3-O-rhamnoside | Flavonols | 10.8 | Neg | 463 | 316 |
| Quercetin-3-O-rutinoside | Flavonols | 10.8 | Neg | 609 | 300 |
| Quercetin-3-O-galactoside | Flavonols | 11.0 | Neg | 463 | 300 |
| Quercetin-3-O-glucoside | Flavonols | 11.2 | Neg | 463 | 300 |
| Luteolin-7-O-glucoside | Flavones | 11.6 | Neg | 447 | 285 |
| Kaempferol-3-O-rutinoside | Flavonols | 11.8 | Neg | 593 | 285 |
| Dihydrokaempferol | Dihydroflavonols | 12.0 | Neg | 287 | 125 |
| Kaempferol-3-O-glucoside | Flavonols | 12.1 | Neg | 447 | 285 |
| Quercetin-3-O-rhamnoside | Flavonols | 12.2 | Neg | 447 | 300 |
| Kaempferol-7-O-neohesperidoside | Flavonols | 12.3 | Neg | 593 | 285 |
| Apigenin-7-O-glucoside | Flavones | 12.8 | Neg | 431 | 268 |
| Quercetin-4′-O-glucoside | Flavonols | 13.0 | Neg | 463 | 301 |
| Myricetin | Flavonols | 13.3 | Neg | 317 | 151 |
| Luteolin-4'-O-glucoside | Flavones | 13.4 | Neg | 447 | 285 |
| Resveratrol | Stilbenes | 13.6 | Neg | 227 | 143 |
| Resveratrol-(4-hydroxyphenyl-13C6) | IS | 13.6 | Neg | 233 | 149 |
| 4-Hydroxy-6-methylcoumarin | IS | 14.0 | Neg | 175 | 131 |
| Quercetin | Flavonols | 15.5 | Neg | 301 | 151 |
| Luteolin | Flavones | 15.9 | Neg | 285 | 133 |
| Naringenin | Flavanones | 16.8 | Neg | 271 | 151 |
| Kaempferol | Flavonols | 17.6 | Neg | 285 | 187 |
| Apigenin | Flavones | 17.7 | Neg | 269 | 117 |
| Flavone | Flavones | 20.2 | Pos | 223 | 77 |
| Flavanone | Flavanones | 22.0 | Pos | 225 | 121 |
IS represents an internal standard.
Compounds without chemical standards are indicated with an *.
Table 2. Proanthocyanidin characteristics in single ion monitoring (SIM), including retention time and molecular ion in positive mode.
| Composition | Sub-class | Retention time (min) | Mode | Molecular Ion (m/z) |
|---|---|---|---|---|
| GGC_I | Proanthocyanidins | 4.7 | Pos | 899.7 |
| GC_I | Proanthocyanidins | 4.9 | Pos | 595.6 |
| GGGC_I | Proanthocyanidins | 5 | Pos | 1204.8 |
| GGG | Proanthocyanidins | 5.3 | Pos | 915.6 |
| GGC_II | Proanthocyanidins | 5.4 | Pos | 899.7 |
| GCC_I | Proanthocyanidins | 5.5 | Pos | 883.7 |
| CC-gallate | Proanthocyanidins | 5.5 | Pos | 731.7 |
| GGGC_II | Proanthocyanidins | 5.6 | Pos | 1204.8 |
| GGCC_I | Proanthocyanidins | 5.6 | Pos | 1187.8 |
| GGC_III | Proanthocyanidins | 5.7 | Pos | 899.7 |
| GGGCC_I | Proanthocyanidins | 5.7 | Pos | 1492.2 |
| GC_II | Proanthocyanidins | 5.9 | Pos | 595.6 |
| GGCC_II | Proanthocyanidins | 6.2 | Pos | 1187.8 |
| GCCC_I | Proanthocyanidins | 6.3 | Pos | 1171.8 |
| GGGC_III | Proanthocyanidins | 6.3 | Pos | 1204.8 |
| GCC_II | Proanthocyanidins | 6.4 | Pos | 883.7 |
| GCC_III | Proanthocyanidins | 6.5 | Pos | 883.7 |
| ±-Catechin-2,3,4-13C3 | IS | 6.6 | Pos | 294.2 |
| GGGCC_II | Proanthocyanidins | 6.9 | Pos | 1492.2 |
| GCCCC_I | Proanthocyanidins | 6.9 | Pos | 1460.1 |
| CCCC_I | Proanthocyanidins | 7.3 | Pos | 1155.9 |
| GCCC_II | Proanthocyanidins | 7.3 | Pos | 1171.8 |
| CCCCC | Proanthocyanidins | 7.9 | Pos | 1444.1 |
| GCCCC_II | Proanthocyanidins | 7.9 | Pos | 1460.1 |
| CCCC_II | Proanthocyanidins | 8.5 | Pos | 1155.9 |
IS, C, and G stand for internal standard, catechin/ epicatechin and gallocatechin/ epigallocatechin, respectively.
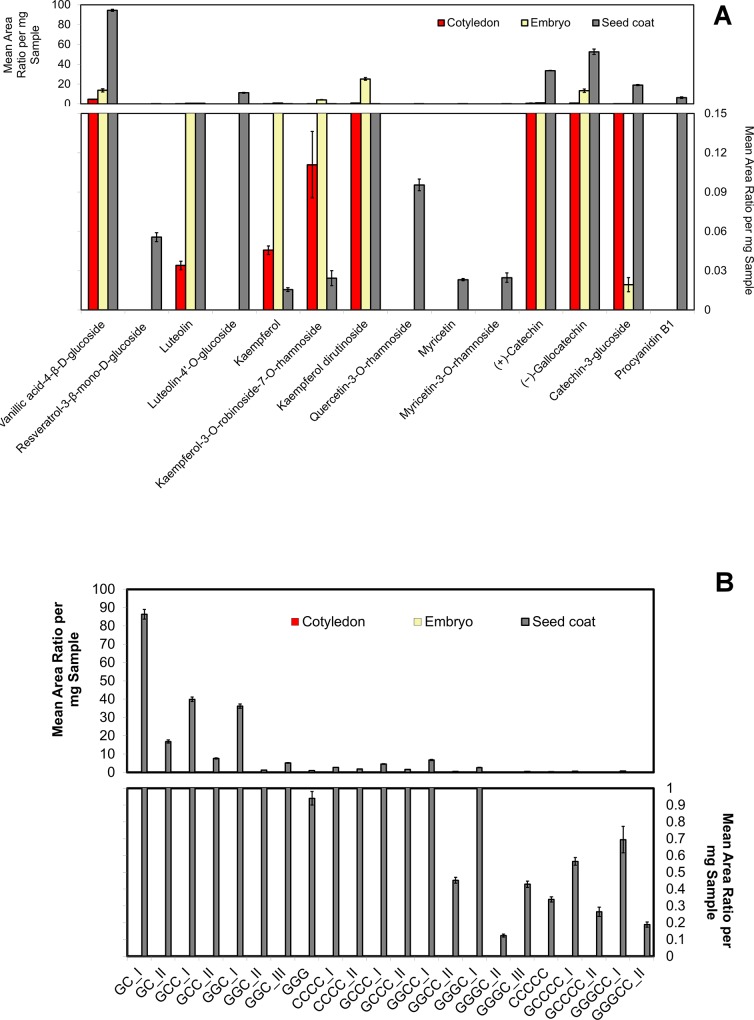
In a preliminary experiment, analysis of variance showed no significant differences between the RILs within either the same phenotypic groups of normal brown opaque seed coats (genotype Ggc Tgc Tan) or gray translucent zero-tannin seed coats (genotype Ggc tan) for most of the analyzed phenolic compounds (S1 and S2 Tables). In a second preliminary experiment, the phenolic profiles of the three seed fractions (cotyledon, seed coat, and embryo) of the gray seed coat of CDC Maxim were significantly different (Fig 2A and 2B). Vanillic acid-4-ß-D-glucoside, luteolin, kaempferol glycones and aglycone, and flavan-3-ols (including catechin, gallocatechin, and catechin-3-glucoside) were detected in all three seed fractions, but resveratrol-3-ß-mono-D-glucoside, luteolin-4'-O-glucoside, quercetins, myricetins, and oligomers of proanthocyanidins (i.e., dimers, trimers, tetramers, and pentamers) were detected only in the seed coat fraction.
Fig 2.
Mean area ratio per mg sample obtained for different sub-classes of phenolic compounds in (A) selected reaction monitoring (SRM) mode and (B) single ion monitoring (SIM) mode in cotyledon, embryo, and seed coat fractions of lentils with genotype Ggc Tgc Tan; C and G indicate catechin/ epicatechin and gallocatechin/ epigallocatechin, respectively.
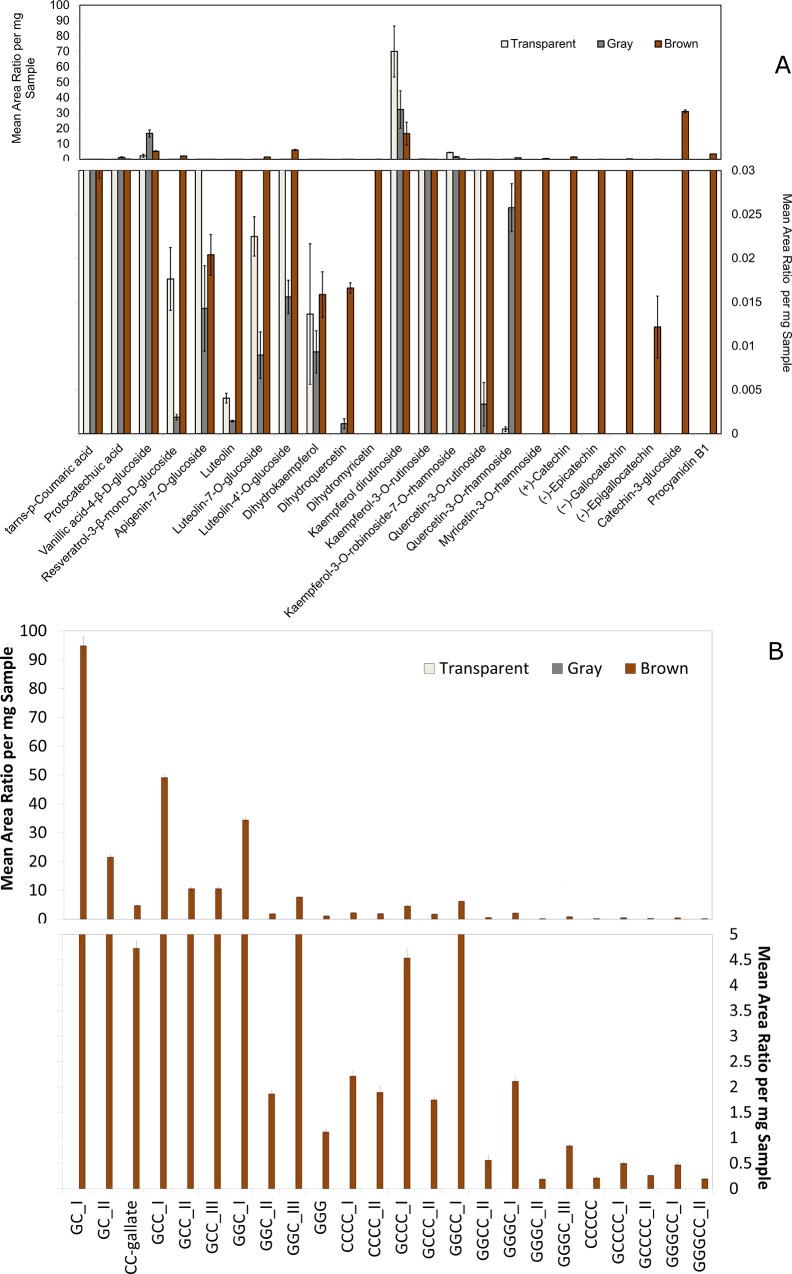
Based on the results of the two preliminary tests, further investigations into the phenolic profile for seed coats that were normal brown opaque (Ggc Tgc Tan), gray translucent zero-tannin (Ggc tan), and transparent zero-tannin (ggc tan) were conducted. Among the phenolic acids, trans-p-coumaric acid, protocatechuic acid, and vanillic acid-4-β-D-glucoside were detected in all three phenotypes (Fig 3A). Resveratrol-3-β-mono-D-glucoside and flavones, including apigenin-7-O-glucoside and luteolin aglycone and glycones, were found in all three seed coat types. Among the dihydroflavonols, dihydrokaempferol was found in all three seed coat phenotypes while dihydroquercetin was predominantly found in the brown opaque and to a lesser amount in the gray translucent seed coats. Dihydromyricetin was detected only in the gray zero-tannin phenotype. Kaempferol glycones were detected in all three seed coat phenotypes. Quercetin-3-O-rutinoside was detected at a low level in gray phenotypes, while quercetin-3-O-rhamnoside was at a very low level in transparent zero-tannin. Myricetin-3-O-rhamnoside, however, was found only in the brown opaque phenotype. Flavan-3-ols including catechin, epicatechin, gallocatechin, epigallocatechin, and catechin-3-glucoside were observed only in brown opaque seed coats. Similar results were observed for proanthocyanidin dimers, trimers, tetramers, and pentamers (Fig 3A and 3B).
Fig 3.
Mean area ratio per mg sample obtained for different sub-classes of phenolic compounds in (A) SRM mode and (B) SIM mode for transparent, gray translucent, and brown opaque lentil seed coats; C and G stand for catechin/ epicatechin and gallocatechin/ epigallocatechin, respectively.
Molecular Analysis
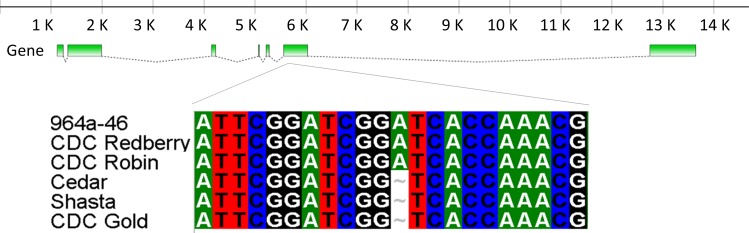
The SNP marker LcC01900p336 was found in a contig that was homologous to the 3’ end of the A gene of pea. It was polymorphic between the parents of LR-30 and it co-segregated with the seed coat phenotype in the segregating RILs (S3 Table). When tested on a panel of 96 lines, however, the genotyping results did not correlate with the phenotypes (data not shown), suggesting it is not the causative mutation and is simply genetically linked in the LR-30 population. Sequencing through the exonic regions of this gene in multiple tan and Tan lines revealed a common SNP in all three tan lines that was not found in the Tan lines (Fig 4). The gene consisted of seven exons and this SNP, at position 343 in exon 6, introduces a premature STOP codon that would result in a truncated protein and a non-functioning enzyme. It should be noted that the mutation in pea that causes the white flower character is caused by an SNP in the splice site at the end of Exon 6, ~165 bp after this deletion. The KASP assay LcZT-Exon6p343, designed to test for this SNP, consistently identified all tan lines and demonstrated that none of the Tan lines have the variant allele in the mapping population LR-30 (S3 Table and S1 Fig) and in the diversity panel (S4 Table and S2 Fig).
Fig 4. Structure of LcubHLH highlighting the region of the variant related to tan.
The tan lines (Cedar, Shasta and CDC Gold) all have a deletion relative to the Tan lines (964a-46, CDC Redberry, and CDC Robin). The gene structure and the sequence alignment were obtained by FancyGene [28] tool and BioEdit [29] alignment software, respectively.
Discussion
Phenolic compounds are produced through the actions of numerous regulatory genes and TFs in the phenylpropanoid pathway. These compounds fulfill different roles including seed pigmentation for plants and confer health benefits to humans who eat the seeds [2–6]. We used a combination of biochemical and genetic approaches to investigate the phenylpropanoid pathway to elucidate what is responsible for the lack of seed coat pigmentation in zero-tannin (tan) lentil phenotypes. To accomplish this, we compared seed coats from brown opaque (Ggc Tgc Tan), gray translucent zero-tannin (Ggc tan), and transparent zero-tannin (ggc tan) phenotypes.
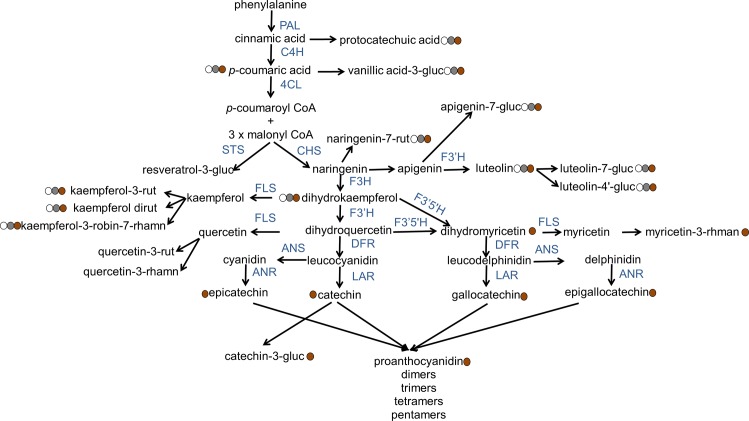
The most obvious difference between the Tan and tan genotypes was the presence of dihydromyricetin, myricetin-3-O-rhmanoside, flavan-3-ols, and proanthocyanidin oligomers in the brown lines and the absence of these in the zero-tannin phenotypes (Fig 3A and 3B). We layered our phenolic compound profile results on the pathway suggested in previous literature [9–12] and present them as a putative biochemical pathway in Fig 5. Dihydromyricetin can be produced by F3’5’H from dihydroquercetin and/ or dihydrokaempferol (Fig 5). Myricetin, gallocatechin/ epigallocatechin (from flavan-3-ols), and several proanthocyanidins should be produced from dihydromyricetin in subsequent steps; however, none of these phenolic compounds were detected in the zero-tannin phenotypes. This shows that the phenylpropanoid pathway in these phenotypes is being blocked at the point where F3’5’H acts. Furthermore, catechin/ epicatechin requires dihydroquercetin as a precursor, and therefore the phenylpropanoid pathway should also be blocked at the location of DFR activity. In Brassica carinata seeds, dihydrokaempferol, dihydroquercetin, and trace amounts of dihydromyricetin accumulate in yellow-seeded (i.e., transparent seed coat) phenotypes, while proanthocyanidins are observed only in brown-seeded phenotypes [30]. The level of mRNA for flavanone-3-hydroxylase (F3H) and flavonoid-3’-hydroxylase (F3’H) is similar between dark and transparent seed coats of B. rapa. However, the amounts of mRNA for DFR, anthocyanidin synthase (ANS), and anthocyanidin reductase (ANR) are not statistically significant in transparent seed coat phenotypes [31]. Arabidopsis thaliana tt3 mutant seeds have transparent seed coats, and visible anthocyanidins or proanthocyanidins are not detected in the tt3 mutant because it lacks DFR mRNA [32]. Strong down-regulation of genes such as ANR and ANS lead to reduced amounts of proanthocyanidins and anthocyanidins and a transparent seed coat in Medicago truncatula [33].
Fig 5. Putative biochemical pathway of phenolic compounds in lentil seed coats, as well as whether or not the final product of different branches of phenylpropanoid are observed in Tan and/ or tan genotypes.
Abbreviations: PAL, phenylalanine ammonia lyase; C4H, cinnamic acid 4-hydroxylase; 4CL, 4-coumaric acid:CoA ligase; STS, stilbene synthase; CHS, chalcone synthase; F3H, flavanone-3-hydroxylase; F3’H, flavonoid-3’-hydroxylase; F3’5’H, flavonoid-3’,5’-hydroxylase; FLS, flavonol synthase; DFR, dihydroflavonol reductase; ANS, anthocyanidin synthase; LAR, leucoanthocyanidin reductase; ANR, anthocyanidin reductase; gluc, glucoside; rut, rutinoside; robin, robinoside; rhamn, rhamnoside. Filled circles represent the transparent, gray, and brown seed coat colours. Information with respect to the pathway originates from [9–12].
Our molecular analyses confirm that tan is most likely a bHLH, orthologous to the A-gene in pea. The LcZT-Exon6p343 allele found in tan lentil genotypes introduces a premature STOP codon that prevents the expression of a full copy of bHLH. As tan is epistatic to Tgc, the tan ground colour is not observed in Ggc Tgc tan or ggc Tgc tan genotypes. The gene MtTT8 in M. truncatula (which is a homologous bHLH) controls pranthocyanidin- and anthocyanidin-related genes such as ANR and ANS [33]. A large insertion mutation in BrTT8 results in transparent phenotype in B. rapa. This bHLH controls the expression of ANS and ANR [31]. The allele tt3 (DFR) in A. thaliana seeds with transparent seed coats is controlled by a group of TFs including bHLHs such as TT8 [34]. TT2 (R2R3-MYB protein) and TTG1 (WDR protein) control DFR, showing that they also interact to control the phenylpropanoid pathway genes. A ternary MBW complex has been proposed for controlling the late sections of the phenylpropanoid pathway [34]. Therefore tan, as a bHLH part of this MBW complex, interacts with the regulatory genes in the phenylpropanoid pathway starting from F3’5’H and DFR.
All of the analyzed phenolic acids are found in all three lentil seed coat colours (Fig 3A). Among the flavonols, kaempferols are found in all three seed coat phenotypes tested. The aglycone and glycones of kaempferols are also observed in all three seed fractions (i.e., embryo, cotyledon, seed coat) (Fig 2A). However, the remaining flavonols, including quercetins and myricetins, are detected only in the seed coat (Fig 2A). Our analysis did not detect dihydroquercetin in the transparent phenotype; however, quercetin glycones are found in all three lentil seed coat colours. This suggests that dihydroquercetin should be present in the transparent seed coat. Because the signal intensity of the dihydroquercetin in the brown seed coat was observable but weak, this compound appears to be in low abundance and likely below the low detection limit in the transparent lentil phenotype.
The phenylpropanoid pathway affects plant characteristics other than pigmentation, including protection of the plant against stresses. Although seed coat phenolics can provide a good barrier against pathogens, the embryo and cotyledons need to be protected by chemical defense materials such as phenolic compounds when the seed starts germinating and the barrier ruptures [35]. Dueñas et al. (2002) reported that catechin and various phenolic acids were present in lentil cotyledons [18]. However, they did not report a variety of flavonoids in cotyledons of lentils. Our study detected a diversity of phenolic compounds, including phenolic acids, flavones, flavonols, and different flavan-3-ols, in cotyledon and embryo fractions. All or some of these compounds may play a role in the protection of the embryo and cotyledons.
Zero-tannin lentils do not change colour during storage [24], likely due to the lack of flavan-3-ols and proanthocyanidins. We previously reported a significant reduction in flavan-3-ols and proanthocyanidins due to polymerization of these compounds in lentils that were stored in the dark for long periods of time [36]. However, phenolic compounds improve seed establishment [37], and as a result their reduction might introduce problems with damage caused by rapid water imbibition during germination [24, 25]. Furthermore, zero-tannin lentils are more susceptible to soil- and seed-borne diseases, a problem that must be circumvented by using seed-applied fungicides [38].
Flavan-3-ols such as catechin and gallocatechin show anti-inflammatory and anti-oxidative activity and have been associated with the reduction of some cardiovascular diseases [39, 40]. Proanthocyanidins are the major antioxidants entering the colon [5], and they may reduce cholesterol [40], inhibit the growth of breast cancer cells [41], and protect the prostate [42]. Zero-tannin lentils cannot provide the health benefits associated with flavan-3-ols and proanthocyanidins; but some health advantages can be achieved. Phenolic acids, flavones, and flavonols show anti-oxidative [2–4], anti-cardiovascular disease [3], anti-cancer [3–6], anti-diabetic [3, 6], and anti-HIV [3, 6] effects. They may also increase the bioavailability of iron [8]. Our new knowledge of the underlying basis of the genotypes and phenotypes of zero-tannin lentil seed coats will be useful for designing future lentil cultivars with improved nutritional profiles.
Materials and Methods
Plant Material
Lentil recombinant inbred line (RIL) population LR-30, which consists of 138 lines, was derived from a cross between the brown seed coat cultivar CDC Robin (genotype Ggc Tgc Tan) and a zero-tannin plant from the breeding line 2670b (genotype Ggc Tgc tan). Both genotypes are homozygous for Tgc and the RILs of this population have either normal brown or gray zero-tannin seed coats based on segregation of the dominant or recessive alleles at the Tan locus. Seed coats of RILs were phenotyped visually and classified as brown opaque (Ggc Tgc Tan) or zero-tannin gray translucent (Ggc Tgc tan).
In a preliminary test, two subsets of 10 RILs of each phenotype were randomly selected for biochemical analysis of the phenolics profile of the lentil seeds. Whole seeds of these 20 RILs were obtained from three biological replicates grown in a randomized complete block design in the field in 2013 at Saskatoon, SK, Canada.
In a second preliminary test, one available gray seed coat normal genotype, CDC Maxim (Ggc tgc Tan), was decorticated and seed coats were separated from cotyledons and embryos [43]. All three seed fractions were similarly analyzed with three technical replicates.
Based on the preliminary analyses, one representative RIL from the Ggc Tgc Tan genotype group (LR-30-76) and one representative from the Ggc Tgc tan genotype group (LR-30-98) were compared with seed coats of a ggc tan genotype (CDC Gold) (Fig 1). CDC Gold has a transparent seed coat that allows its cotyledon colour to be easily observed. Seeds of CDC Gold were also produced in the field in 2013 at Saskatoon.
The seeds of all three genotypes were decorticated to obtain the seed coat fractions that were analyzed using three technical replicates.
Reagents and Standards
Tables 2 and 3 show a complete list of the phenolic compounds analyzed in this study, including sub-classes of phenolic acids, stilbenes, anthocyanidins, flavan-3-ols, proanthocyanidins, flavanones, flavones, dihydroflavonols and flavonols. The majority of the phenolic compounds investigated in this experiment were identified previously (S1 Text) and [43]. Additional standards investigated in this work included protocatechuic acid, 4-hydroxybenzoic acid, chlorogenic acid, trans-p-coumaric acid, trans-ferulic acid, dihydroquercetin, dihydrokaempferol, dihydromyricetin, resveratrol-(4-hydroxyphenyl-13C6), cyanidin-3-O-rhamnoside, and resveratrol. All standards were purchased from Sigma-Aldrich (Missouri, USA) except cyanidin-3-O-rhamnoside, which was purchased from Extrasynthese (Genay, France). The two compounds labelled with an asterisk (*) in Table 1 (catechin-3-glucoside and kaempferol dirutinoside) are not commercially available, but have been previously identified in lentil seed [17, 19] and putatively identified in our previous work (S1 Text) and [43]. The proanthocyanidins in Table 2 did not have commercially available standards, and therefore the order of C’s (catechin or epicatechin) and G’s (gallocatechin or epigallocatechin) is arbitrarily assigned for these oligomers.
Table 3. Mobile phase gradient used in this experiment, where solvent A and B were water: formaic acid (99;1, v/v) and water: acetonitrile: formic acid (9:90:1, v/v/v), respectively.
| Time (min) | A% | B% | Flow (mL/min) |
|---|---|---|---|
| 0 | 99 | 1 | 0.35 |
| 1 | 99 | 1 | 0.35 |
| 21 | 59 | 41 | 0.35 |
| 24 | 40 | 60 | 0.35 |
| 24.1 | 20 | 80 | 0.35 |
| 26 | 20 | 80 | 0.35 |
| 26.1 | 99 | 1 | 0.35 |
| 30 | 99 | 1 | 0.35 |
Sample Preparation
For each replicate, 1000 μL of the extraction solvent (acetone:water, 70:30 v/v) containing the internal standards was added to 250 mg of freeze-dried sample in micro-centrifuge tubes. The internal standards are added to account for changes in the matrix among the cultivars and enables relative quantification [44] to be used when comparing the phenolic profiles among the cultivars. When separate seed fractions (cotyledons, embryos, and seed coats) were analyzed, the extraction solvent and the freeze-dried samples were reduced to 250 μL and 50 mg, respectively. Samples were crushed into a fine paste using a Fast Prep®FP120 (Qbiogene, Inc., Canada) with a maximum of seven consecutive times for 45 s each at a speed setting of 4.0. Samples were shaken for 1 h on a rocking platform at a speed of 1400 rpm. The tubes were centrifuged twice (12,000 rpm for 5 min each) and 100 μL of the supernatant was dried down with a Speed Vac (LABCONCO, Kansas City, USA). Dried samples were then reconstituted in 100 μL methanol: water (10:90, v/v) (S1 Text).
HPLC-MS
Previously optimized chromatographic conditions [43] were applied with some modifications. The LC hardware was an Agilent 1290 UPLC equipped with a G4226A autosampler, a G4220 A binary pump, a G1316 TCC, and a G4212 DAD detector. The column was a Core-shell Kinetex pentafluorophenyl (PFP) (100 mm × 2.1 mm id), with 2.6 μm particle size (Phenomenex, Torrance, CA). The mobile phases were water:formic acid (FA) (99:1, v/v) as solvent A and water:acetonitrile (ACN):FA (9:90:1, v/v/v) as solvent B. The same solvent gradient was employed as previously reported (Table 3) [43]. Although some retention times were slightly earlier than in our previous study, this was readily attributed to the smaller mixing volume of the Agilent 1290 UPLC compared with the Agilent 1100 HPLC. Relative quantification was determined for phenolic compounds (Table 1) using selected reaction monitoring (SRM) and for proanthocyanidins (Table 2) using single ion monitoring (SIM). Peak areas of each analyte were integrated with Thermo Xcalibur 2.1 software and normalized to the peak area of a related internal standard (IS). Values are reported per mg of dry sample.
Statistical Analysis
Analysis of variance and means comparisons of area ratio per mg of sample were done using R software (v. 2.15.3) [45]. Duncan’s multiple range test was used for comparing the means of area ratio per mg samples (95% confidence level).
Molecular Markers
To initially test if the lentil homologue of the A-gene of pea segregated with tan, the nucleotide sequence for the pea gene [GU132941] was used to tBLASTx an in-house collection of 3’ transcript sequences of lentil from which SNPs had been identified [46]. A number of sequences from various lentil lines matched the pea sequence. An alignment of these sequences, using BioEdit [29] alignment software, revealed an SNP (LcC01900p336) located 52 nucleotides downstream from the STOP codon. A KASP assay (LGC Genomics, Hoddesdon, UK) was designed to assay genotypes at this SNP. The allele specific primers were A1 = GAAGGTGACCAAGTTCATGCTGACAAAATCACGTGATGTTGTGACTC and A2 = GAAGGTCGGAGTCAACGGATTGACAAAATCACGTGATGTTGTGACTT. The conserved primer was C1 = AAGCCAATGTGTACCAATGATGTATCATTA. DNA was extracted from a single individual of each LR-30 RIL using a modified CTAB extraction [47]. Assay reaction volume was 10 μL of 50 ng/μL DNA, 2X KASP Reaction Mix, and 0.17 μM KASP Assay Mix (allele-specific primers, A1 and A2, and common primer, C1). PCR amplification was carried out in a StepOnePlusTm Real-Time PCR System (Applied Biosystems, California, US) and fluorescence was analyzed using StepOne Software version 2.1 (Applied Biosystems, California, US).
To identify the putative causal mutation in the gene, the pea sequence was compared to a preliminary assembly of the lentil genome (CDC Redberry v0.3) using tBLASTx to identify the full lentil homologue. Nested primers were designed to span the introns across the full gene and to amplify fragments from several Tan and tan lines. Amplified fragments were run out on a 1% agarose gel, bands were cut out and purified using a Qiagen gel extraction kit (cat.no. 28706), and the resulting DNA sequenced using the Sanger method. Sequences were aligned to the reference genome (CDC Redberry v0.3) and SNPs identified using BioEdit alignment software.
A KASP assay, LcZT-Exon6p343, with
A1 = GAAGGTGACCAAGTTCATGCTGCCCGATGATATTCGGATCGGA,
A2 = GAAGGTCGGAGTCAACGGATTGCCCGATGATATTCGGATCGGT, and
C = GGCCAACAAATGAAAATCTGAGTCCAAAT), was designed for a candidate SNP and used to survey a panel of 96 lentil genotypes (S4 Table) representative of a wide range of seed coat colours and patterns and the zero-tannin cultivars Cedar, Shasta, CDC Zt-4, and CDC Gold.
Supporting Information
(XLSX)
(XLSX)
** and *** denote significance at the 0.01 and 0.001 probability levels, respectively.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Acknowledgments
The authors acknowledge financial assistance from Saskatchewan Pulse Growers and the NSERC Industrial Research Chair program as well as instrumentation provided by Thermo Fisher Scientific (San Jose, CA). We also acknowledge additional support provided by the Health Sciences core mass spectrometry facility and the Pulse Research Crew at the Crop Development Centre, University of Saskatchewan. We would like to particularly thank R. Varma Penmetsa for initially pointing us towards the gene. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors acknowledge financial assistance from Saskatchewan Pulse Growers and the NSERC Industrial Research Chair program as well as instrumentation provided by Thermo Fisher Scientific (San Jose, CA). We also acknowledge additional support provided by the Health Sciences core mass spectrometry facility and the Pulse Research Crew at the Crop Development Centre, University of Saskatchewan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vermeris W, Nicholson R. Phenolic Compound Biochemistry. Dordrecht, The Netherlands: Springer; 2006. [Google Scholar]
- 2.Delgado-Vargas F, Jiménez AR, Paredes-López O. Natural pigments: carotenoids, anthocyanins, and betalains—characteristics, biosynthesis, processing, and stability. Crit Rev Food Sci Nutr 2000;40(3):173–289. 10.1080/10408690091189257 [DOI] [PubMed] [Google Scholar]
- 3.Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini-Rev Med Chem. 2011;11:298–344. [DOI] [PubMed] [Google Scholar]
- 4.Spilioti E, Jaakkola M, Tolonen T, Lipponen M, Virtanen V, Chinou I, et al. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. 2014. 9(4):e94860 10.1371/journal.pone.0094860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. [DOI] [PubMed] [Google Scholar]
- 6.Xiao J, Chen T, Cao H. Flavonoid glycosylation and biological benefits. Biotechnol Adv. 2014. 10.1016/j.biotechadv.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 7.DellaValle DM, Vandenberg A, Glahn RP. Seed coat removal improves iron bioavailability in cooked lentils: studies using an in vitro digestion/Caco-2 cell culture model. J Agric Food Chem. 2013;61:8084–9. 10.1021/jf4022916 [DOI] [PubMed] [Google Scholar]
- 8.Hart JJ, Tako E, Kochian LV, Glahn RP. Identification of black bean (Phaseolus vulgaris L.) polyphenols that inhibit and promote iron uptake by Caco-2 cells. J Agric Food Chem. 2015. 10.1021/acs.jafc.5b00531 [DOI] [PubMed] [Google Scholar]
- 9.Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welcha CR, Wub Q, Simon JE. Recent advances in anthocyanin analysis and characterization. Curr Annal Chem. 2008;4:75–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grignon-Dubois M, Rezzonico B. First phytochemical evidence of chemotypes for the seagrass Zostera noltii. Plants 2012. 2012;1:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaushik P, Andújar I, Vilanova S, Plazas M, Gramazio P, Javier Herraiz F, et al. Breeding vegetables with increased content in bioactive phenolic acids. Molecules. 2015;20:18464–81. 10.3390/molecules201018464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen ØM, Jordheim M. Chemistry of flavonoid-based colors in plants In: Mander LN, Liu HW, editors. Comprehensive Natural Products II: Chemistry and Biology. 3 Oxford: Elsevier; 2010. p. 547–614. [Google Scholar]
- 14.Albert NW, Davies KM, Lewis DH, Zhang H, Montefiori M, Brendolise C, et al. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell. 2014;26:962–80. 10.1105/tpc.113.122069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenberg V, Slinkard AE. Genetics of seed coat color and pattern in lentil. J Hered. 1990;81(6):484–8. [Google Scholar]
- 16.Xu B, Chang SKC. Phenolic substance characterization and chemical and cell-based antioxidant activities of 11 lentils grown in the northern United States. J Agric Food Chem. 2010;58(3):1509–17. 10.1021/jf903532y [DOI] [PubMed] [Google Scholar]
- 17.Aguilera Y, Dueñas M, Estrella I, Hernández T, Benitez V, Esteban RM, et al. Evaluation of phenolic profile and antioxidant properties of pardina lentil as affected by industrial dehydration. J Agric Food Chem. 2010;58(18):10101–8. 10.1021/jf102222t [DOI] [PubMed] [Google Scholar]
- 18.Dueñas M, Hernández T, Estrella I. Phenolic composition of the cotyledon and the seed coat of lentils (Lens culinaris L.). Eur Food Res Technol. 2002;215:478–83. 10.1007/s00217-002-0603-1 [DOI] [Google Scholar]
- 19.Dueñas M, Sun B, Hernández T, Estrella I, Spranger MI. Proanthocyanidin composition in the seed coat of lentils (Lens culinaris L.). J Agric Food Chem. 2003;51(27):7999–8004. 10.1021/jf0303215 [DOI] [PubMed] [Google Scholar]
- 20.López-Amorós ML, Hernández T, Estrella I. Effect of germination on legume phenolic compounds and their antioxidant activity. J Food Comp Anal 2006;19:277–83. [Google Scholar]
- 21.Zhang B, Deng Z, Ramdath DD, Tang Y, Chen PX, Liu R, et al. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on a-glucosidase and pancreatic lipase. Food Chem. 2015;172 862–72. 10.1016/j.foodchem.2014.09.144 [DOI] [PubMed] [Google Scholar]
- 22.Vaillancourt R, Slinkard AE, Reichert RD. The inheritance of condensed tannin concentration in lentil. Can J Plant Sci. 1986;66(2):241–6. 10.4141/cjps86-038 [DOI] [Google Scholar]
- 23.Muehlbauer F, Sarker A. Tannin free lentils: A promising development for specialty use and increased value. Grain Legumes. 2011;56. [Google Scholar]
- 24.Matus A, Slinkard AE, Vandenberg A. The potential of zero tannin lentil In: Janick J, Simon JE, editors. New crops. New York: Wiley; 1993. p. 279–82. [Google Scholar]
- 25.Smýkal P, Vernoud V, Blair MW, Soukup A, Thompson RD. The role of the testa during development and in establishment of dormancy of the legume seed. Front Plant Sci. 2014;5:351 10.3389/fpls.2014.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendel JG. Versuche über Pflanzenhybriden Abhandlungen. 1865:3–47. [Google Scholar]
- 27.Hellens RP, Moreau C, Lin-Wang K, Schwinn KE, Thomson SJ, Fiers MWEJ, et al. Identification of Mendel's white flower character. PLoS ONE. 2010;5(10):e13230 10.1371/journal.pone.0013230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rambaldi D, Ciccarelli FD. FancyGene: dynamic visualization of gene structures and protein domain architectures on genomic loci. Bioinformatics. 2009;25(17):2281–2. 10.1093/bioinformatics/btp381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;(41):95–8. [Google Scholar]
- 30.Marles MAS, Gruber MY, Scoles GJ, Muira AD. Pigmentation in the developing seed coat and seedling leaves of Brassica carinata is controlled at the dihydroflavonol reductase locus. Phytochemistry. 2003. 62:663–72. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Chen L, Hong M, Zhang Y, Zu F, Wen J, et al. A large insertion in bHLH transcription factor BrTT8 resulting in yellow seed coat in Brassica rapa. PLoS ONE. 2012;7(9):e44145 10.1371/journal.pone.0044145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, et al. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995;8(5):659–71. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Chen B, Zhang G, Chen L, Dong Q, Wen J, et al. Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bHLH transcription factor MtTT8. New Phytol. 2016. 10.1111/nph.13816 [DOI] [PubMed] [Google Scholar]
- 34.Appelhagen I, Jahns O, Bartelniewoehner L, Sagasser M, Weisshaar B, Stracke R. Leucoanthocyanidin Dioxygenase in Arabidopsis thaliana: Characterization of mutant alleles and regulation by MYB–BHLH–TTG1 transcription factor complexes. Gene. 2011;484:61–8. 10.1016/j.gene.2011.05.031 [DOI] [PubMed] [Google Scholar]
- 35.Gallery RE, Moore DJP, Dalling JW. Interspecific variation in susceptibility to fungal pathogens in seeds of 10 tree species in the neotropical genus Cecropia. J Ecol. 2010;98:147–55. [Google Scholar]
- 36.Mirali M, Purves RW, Vandenberg A. Phenolic profiling of green lentil (Lens culinaris Medic.) seeds subjected to long-term storage. Eur Food Res Technol. 2016:In press. [Google Scholar]
- 37.Randhir R, Shetty K. Light-mediated fava bean (Vicia faba) response to phytochemical and protein elicitors and consequences on nutraceutical enhancement and seed vigour. Process Biochem. 2003;38 945–52. [Google Scholar]
- 38.Matus A, Slinkard AE. Effect of fungicidal seed treatment on zero-tannin lentil. Lens Newsletter. 1993;20:46–50. [Google Scholar]
- 39.Gonza´ Lez R, Ballester I, Lo´ Pez-Posadas R, Sua´ Rez MD, Zarzuelo A, Marti´Nez-Augustin O, et al. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci. 2011;51:331–62. [DOI] [PubMed] [Google Scholar]
- 40.Toh JY, Tan VMH, Lim PCY, Lim ST, Chong MFF. Flavonoids from fruit and vegetables: a focus on cardiovascular risk factors Curr Atheroscler Rep 2013;15:368 10.1007/s11883-013-0368-y [DOI] [PubMed] [Google Scholar]
- 41.Ramljak D, Romanczyk LJ, Metheny-Barlow LJ, Thompson N, Knezevic V, Galperin M, et al. Pentameric procyanidin from Theobroma cacao selectively inhibits growth of human breast cancer cells. Mol cancer Ther. 2005;4:537–46. 10.1158/1535-7163.MCT-04-0286 [DOI] [PubMed] [Google Scholar]
- 42.Lei Y, Ren X, Chen J, Liu D, Ruan J. Protective effects of grape seed-derived procyanidin extract against carrageenan-induced abacterial prostatitis in rats. J Funct foods. 2014;7:416–24. [Google Scholar]
- 43.Mirali M, Ambrose SJ, Wood SA, Vandenberg A, Purves RW. Development of a fast extraction method and optimization of liquid chromatography–mass spectrometry for the analysis of phenolic compounds in lentil seed coats. J Chromatogr B. 2014;969:149–61. [DOI] [PubMed] [Google Scholar]
- 44.Lei Z, Huhman DV, Sumner LW. Mass spectrometry strategies in metabolomics. J Biol Chem. 2011;286(29):25435–42. 10.1074/jbc.R111.238691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2013. Available from: http://www.R-project.org/. [Google Scholar]
- 46.Sharpe AG, Ramsay L, Sanderson L-A, Fedoruk MJ, Clarke WE, Li R, et al. Ancient orphan crop joins modern era: gene-based SNP discovery and mapping in lentil. BMC Genomics. 2013;14(192). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
** and *** denote significance at the 0.01 and 0.001 probability levels, respectively.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.