Fig. 2.
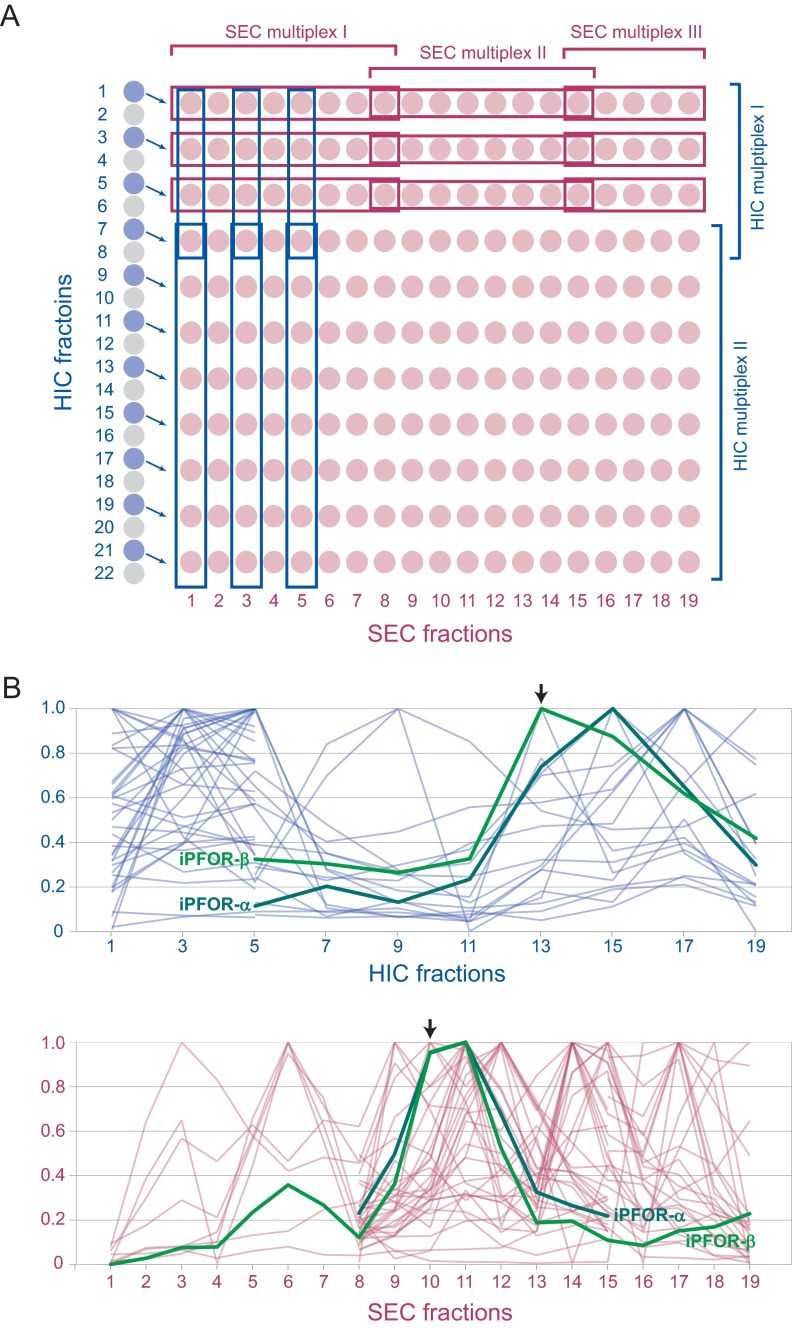
Two dimensional iTRAQ labeling reveals elution profiles in SEC and HIC dimensions. A, Left are shown 22 fractions eluted from a single HIC column. Every other fraction (11 blue disks) was separated on an SEC column, each producing 19 SEC fractions (red disks). The resulting total of 11 × 19 = 228 SEC fractions were digested with trypsin and each digested sample split into several portions to be used for mapping protein elution across the SEC and HIC dimensions (see Experimental Procedures). Two or more portions of each fraction were labeled with an iTRAQ reagent and combined with other fractions labeled with different isobaric iTRAQ reagents to form multiplexes. Multiplexes of up to 8 fractions are allowed by iTRAQ, and thus several multiplexes are required to determine the elution profiles across each column. A common “joint” fraction was included in adjacent multiplexes. Fractions were combined to form multiplexes that track protein elution along the SEC dimension (horizontal) and, separately, along the HIC dimension (vertical). For simplicity only three joined series of multiplexes are shown for each dimension, but from a single HIC column typically 10 joined series would cover the HIC dimension and 10–12 the SEC dimension. B, The iTRAQ elution profiles of proteins across the HIC dimension (top) and the SEC dimension (bottom) are shown. Only one joined series is shown for each dimension out of the larger number of series obtained for every HIC column run and its associated SEC fractions. The black arrows indicate the particular HIC fraction that was separated to produce the SEC profiles and the SEC fractions that were joined into multiplexes to generate profiles of a subset of the proteins eluting on the HIC dimension. The profiles for the alpha and beta subunits of indolepyruvate ferredoxin oxidoreductase (DVU1950 and DVU1951) are shown in bold green. The profiles of all other proteins detected are shown in red (SEC dimension) and blue (HIC dimension).