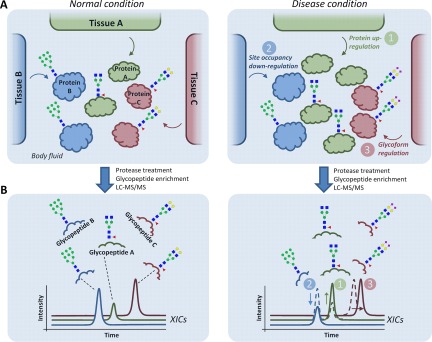
Fig. 3.
Three fundamental levels of molecular dysregulation of multiple tissues contributing to an altered secreted N-glycoproteome during disease. A, Hypothetical example illustrating three sources of dysregulation: 1) protein level (green), 2) site occupancy (blue), or 3) glycosylation micro-heterogeneity (red) from three separate tissues (Tissue A-C) contributing to a joint secreted N-glycoproteome (Protein A-C) in a body fluid derived from disease (right) and 'normal' healthy (left) condition. B, After proteolysis and enrichment, the altered abundance of the resulting glycopeptides can be detected using LC-MS/MS-based label-free quantitative glycoproteomics as shown by color-coded traces representing extracted ion chromatograms (XICs). However, establishing which of the three mechanisms causes the glycopeptide alterations for the detected glycoproteins may be challenging solely with glycopeptide analysis, especially in glycoproteomes arising from multiple tissues. Parallel quantitative proteomics and “deglycoproteomics” (detection of formerly occupied N-sites) of the same samples can assist in this task.