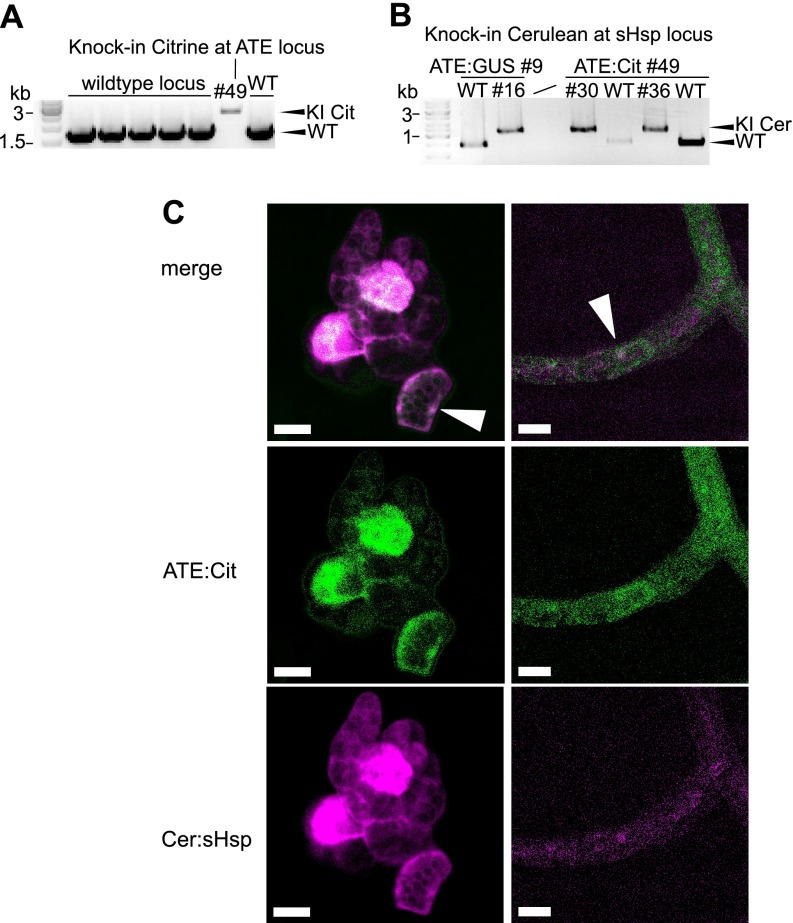
Fig. 3.
Generation of FRET moss (Physcomitrella patens) reporter lines via knock-in. Fluorescent protein tags were introduced into the endogenous ATE gene and sHsp17.2a gene via homologous recombination. A, PCR using genomic DNA of wildtype (WT) and transgenic lines, validating the single integration of Citrine knock-in (KI) construct at the ATE locus using primer flanking the homologous regions used for targeted knock-in (see supplemental Fig. S2 and Material and Methods for additional information). B, PCR using genomic DNA, validating the integration of the Cerulean knock-in construct at the sHsp17.2a locus in two different transgenic background lines. Stable transgenic lines for both the ATE:Citrine and the Cerulean:sHsp17.2a construct were used for FRET analyses, whereas lines with a single fluorophore-tagged protein served as reference lines for spectral imaging and linear unmixing of fluorophore channels using a confocal microscope. C, Exemplary confocal images of the FRET line #36 after linear unmixing of the Citrine and Cerulean signals (see Material and Methods). ATE:Citrine and Cerulean:sHsp17.2a co-localize in several protonema cells, with a low abundance in the cytosol and a higher abundance in the nucleus (arrows). In young gametophores, fluorescence signal was strongest in the single meristematic cell of moss shoots. Scale bars: 20 μm.