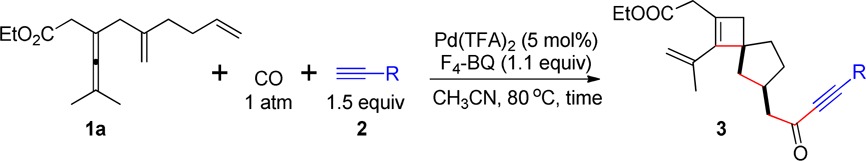
Table 1. Scope of Terminal Alkynesa.
| entry | R | time (h) | yield of 3 (%)b |
|---|---|---|---|
| 1 | Ph | 6 | 75 (3a) |
| 2 | 2-MeOC6H4 | 6 | 72 (3ab) |
| 3 | 3-MeOC6H4 | 6 | 74 (3ac) |
| 4 | 4-MeC6H4 | 6 | 79 (3ad) |
| 5 | 4-FC6H4 | 6 | 64 (3ae) |
| 6 | 4-BrC6H4 | 6 | 66 (3af) |
| 7 | 4-CF3C6H4 | 6 | 77 (3ag) |
| 8 | 2-thiophenyl | 6 | 83 (3ah) |
| 9 | 3-thiophenyl | 6 | 71 (3ai) |
| 10 | Cy | 10 | 66 (3aj) |
| 11 | cinnamyl | 10 | 70 (3ak) |
| 12c | TMS | 15 | 79 (3al) |
The reaction was conducted in MeCN at 80 °C using 1a (0.2 mmol), 2 (1.5 equiv), F4-BQ (1.1 equiv) in the presence of Pd(TFA)2 (5 mol %).
Isolated yield.
TMS-acetylene (3.0 equiv) was used.
