Abstract
FBXW7 is a ubiquitin ligase that mediates ubiquitylation of oncoproteins, such as c‐Myc, cyclin E, Notch and c‐Jun. FBXW7 is a known tumor‐suppressor gene, and mutations in FBXW7 have been reported in various human malignancies. In this study, we examined the sequences of the FBXW7 and p53 genes in 57 ovarian cancer clinical samples. Interestingly, we found no FBXW7 mutations associated with amino acid changes. We also investigated FBXW7 expression levels in 126 epithelial ovarian tumors. FBXW7 expression was negatively correlated with the malignant potential of ovarian tumors. That is to say, FBXW7 expression levels in ovarian cancer samples were significantly lower than those in borderline and benign tumors (P < 0.01). FBXW7 expression levels in serous carcinoma samples were the lowest among four major histological subtypes. In addition, p53‐mutated ovarian cancer samples showed significantly lower levels of FBXW7 expression compared with p53 wild‐type cancer samples (P < 0.001). DNA methylation arrays and bisulfite PCR sequencing experiments revealed that 5′‐upstream regions of FBXW7 gene in p53‐mutated samples were significantly higher methylated compared with those in p53 wild‐type samples (P < 0.01). This data indicates that p53 mutations might suppress FBXW7 expression through DNA hypermethylation of FBXW7 5′‐upstream regions. Thus, FBXW7 expression was downregulated in ovarian cancers, and was associated with p53 mutations and the DNA methylation status of the 5′‐upstream regions of FBXW7.
Keywords: FBXW7, methylation, mutation, ovarian cancer, p53
In 2008, an estimated 225 500 women were diagnosed with ovarian cancer and 140 200 women died from this disease worldwide.1 In that year, ovarian cancer was the eighth most common type of cancer and the seventh most common cause of cancer‐related death among women. In Japan, the number of deaths due to ovarian cancer has grown from 4006 in 1996, to 4435 in 2006, and to 4705 in 2011, making ovarian cancer the most lethal gynecological cancer.2
The F‐box protein FBXW7 (also known as Archipelago, hAGO, hCDC4) is a substrate‐recognition subunit of an SCF ubiquitin ligase complex. It interacts with substrates undergoing ubiquitylation and mediates the process. Substrates of FBXW7, such as c‐Myc, cyclin E, Aurora A, Notch and c‐Jun, are positive regulators of the cell cycle. Therefore, the FBXW7 gene is considered to be a tumor‐suppressor gene.3, 4, 5, 6 Mao and colleagues reported that murine Fbxw7 was a p53‐dependent haplo‐insufficient tumor suppressor gene and that dysfunction of both p53 and Fbxw7 contributed to carcinogenesis.7 Fbxw7 induces proliferating cells to exit from the cell cycle by triggering the degradation of c‐Myc. Thus, inactivation of Fbxw7 sustains continuous cell cycling (essential for carcinogenesis). This abnormal cell‐cycling is censored by checkpoint activation and eventually restrained by p53 activation. Thus, if both p53 and Fbxw7 are dysfunctional, cancer can develop. Indeed, T‐cell lymphoma develops in T cell‐specific Fbxw7 knockout mice, and T‐cell acute lymphoblastic lymphoma develops in bone marrow‐specific Fbxw7 knockout mice. p53 inactivation in Fbxw7 knockout mice promotes the onset of intestinal cancers in addition to lymphomas.8, 9, 10
Mutations in the FBXW7 gene have been reported in many human malignancies, and the frequency of FBXW7 mutations in human cancers has been estimated to be approximately 6%.11 For example, FBXW7 mutation rates in cholangiocarcinoma, T‐cell acute lymphocytic leukemia and endometrial carcinoma were reported to be 35%, 31% and 16%, respectively.11, 12, 13 However, FBXW7 mutations are infrequent in ovarian cancer.14, 15
The FBXW7 gene encodes three transcripts (FBXW7α, −β and −γ) that are produced by alternative splicing. Each mRNA consists of an isoform‐specific first exon linked to 10 shared exons, generating three protein isoforms that differ only at their N termini.3 This genomic organization is highly conserved in mammals. Each isoform occupies a distinct subcellular location. FBXW7α is found in the nucleoplasm, FBXW7β is localized to the cytoplasmic membrane and FBXW7γ is found in the nucleolus.16, 17 Fbxw7α is expressed at much higher levels than Fbxw7β or Fbxw7γ. Fbxw7α is ubiquitously expressed at high levels, whereas Fbxw7β expression is detected at high levels in the brain, and Fbxw7γ expression is limited to cardiac muscles and skeletal muscles. Each of the three isoforms is thought to have its own promoter and to be under isoform‐specific transcriptional control. Although Fbxw7β is reported to be a transcriptional target of p53,18 the mechanisms regulating Fbxw7α/γ expression remain uncharacterized.
Low expression of FBXW7 is associated with clinicopathological background and prognosis in gastric cancer, colorectal cancer, breast cancer and glioma.19, 20, 21, 22 The mechanisms that regulate FBXW7 expression in cancers are unclear. However, one study has demonstrated that the methylation status of the FBXW7β promoter is inversely correlated with the expression level of FBXW7 in breast cancer.23 In addition, some reports have suggested that microRNA regulate FBXW7 transcript expression in colorectal cancer, esophageal cancer and gastric cancer.24, 25, 26
In the present study, we examined FBXW7(α) mutations in clinical samples of ovarian cancer (n = 57) and gene expression in ovarian tumor clinical samples (n = 126). Mutations of FBXW7 were rare in ovarian cancers and FBXW7 expression levels in ovarian cancers were significantly lower than those in borderline and benign tumors. We also investigated the correlation between p53 mutation status and FBXW7 expression. FBXW7 expression was significantly lower in the p53 mutation group than that in the p53 wild‐type group. In addition, we analyzed the methylation status of the 5′‐upstream regions of FBXW7. DNA methylation arrays and bisulfite sequencing revealed the hypermethylation of FBXW7 5′‐upstream regions in p53‐mutated ovarian cancer samples. Thus, the FBXW7 expression level would be affected by p53 mutations through promoter hypermethylation, which might contribute to the acquisition of the malignant phenotype in ovarian tumors.
Materials and Methods
Ovarian cancer tissues
Ovarian tumor specimens from 126 female patients who were treated at Kyushu University Hospital between 2003 and 2010 were included in the present study. Tumors were histologically characterized as serous (benign, 6; borderline malignancy, 9; carcinoma, 26), mucinous (benign, 11; borderline malignancy, 16; carcinoma, 15), clear cell (borderline malignancy, 1; carcinoma, 25), or endometrioid (carcinoma, 17). The median age of the patients was 55 years old (range 22–79). Patients who had undergone neoadjuvant chemotherapy were excluded from the study. Informed consent was obtained from all patients prior to enrollment in the study. The ethics committee of Kyushu University Graduate School approved the study protocol.
Resected tumor tissues were immediately cut, frozen in liquid nitrogen, and kept at −80°C until RNA and DNA extraction. Total RNA was extracted from tissue specimens using an ISOGEN Kit (NIPPON GENE, Tokyo, Japan). Total RNA (1 μg) was reverse transcribed to cDNA using ReverTra Ace (Toyobo, Osaka, Japan), according to the manufacturer's protocol. Genomic DNA was extracted from frozen specimens using standard phenol/chloroform methods.
Mutation analysis
The FBXW7α sequence was amplified using cDNA and sequencing primers (Table S1). PCR was carried out with PrimeSTAR HS DNA Polymerase (Takara Bio, Shiga, Japan). Likewise, genomic DNA samples were used as templates to PCR amplify exons 4–9 of the p53 gene with primers derived from intronic sequences (Table S1). Thermal cycling parameters were as follows: initialization for 5 min at 98°C followed by 40 cycles of denaturation at 98°C for 10 s, annealing at 58°C for 10 s, and elongation at 72°C for 1 min. These PCR products were electrophoresed on 1.5% agarose gels containing ethidium bromide and purified with an Illustra GFX PCR DNA and Gel Band Purification Kit (GE Healthcare, Buckinghamshire, UK). Purified PCR products were sequenced using a Big‐Dye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA) and an ABI3130xl sequencer (Applied Biosystems, Foster City, CA, USA).
Real‐time quantitative reverse transcription–PCR (qRT‐PCR)
Real‐time qPCR was performed using an Applied Biosystems 7500 Real‐Time PCR System in a 20‐μL reaction volume with SYBR Premix Ex Taq (Takara Bio). Each reaction was carried out under the following conditions: initialization for 30 s at 95°C followed by 40 cycles of 5 s at 95°C for denaturation and 34 s at 60°C for annealing and elongation. The expression of FBXW7 mRNA is presented as the relative copy number normalized to that of glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) mRNA. The PCR primer sequences were as follows: FBXW7α, forward 5′‐TTCACCAACTCTCCTCCCCATT‐3′ and reverse 5′‐GCTGAACATGGTACAAGCCCA‐3′, GAPDH, forward 5′‐GCAAATTCCATGGCACCGT‐3′ and reverse 5′‐TCGCCCCACTTGATTTTGG‐3′.
DNA methylation array
We performed DNA methylation array analysis to evaluate the methylation status of ovarian cancer specimens with Infinium HumanMethylation450 BeadChips (Illumina, San Diego, CA, USA) following by an Illumina Infinium HD MethylationAssay, according to the manufacturer's instructions. The gDNA (1500 ng) was bisulfite‐converted using an Epitect Plus DNA Bisulfite Kit (Qiagen, Hilden, Germany) for use in an Infinium HumanMethylation450 assay. Bisulfite‐converted DNA (300 ng) was used in the whole‐genome amplification reaction. After amplification, the DNA was fragmented enzymatically, precipitated and re‐suspended in hybridization buffer. Fragmented DNA was dispensed onto a HumanMethylation450 BeadChip, and hybridization was performed in a hybridization oven for 20 h. After hybridization, the array was processed through the primer detection step of a single‐base extension reaction. Finally, BeadChips were coated and then imaged on an Illumina iScan. The methylation level of each CpG locus was calculated using GenomeStudio Methylation Module software version 1.0. (Illumina, San Diego, CA, USA) in which the methylation β‐value (β‐value = intensity of the methylated allele (M)/intensity of the unmethylated allele (U) + intensity of the methylated allele (M) + 100) ranged from zero in the case of completely unmethylated loci to one in the case of complete methylation.
Bisulfite sequencing
Bisulfite PCR was carried out with EX Taq Polymerase (Takara Bio). Thermal cycling parameters were as follows: initial denaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C for 30 s, annealing at 57°C for 30 s, and extension at 72°C for 30 s, followed by a final extension at 72°C for 2 min. Primer sets for bisulfite PCR were as follows: biFBXW7 forward 5′‐TGTTGTAGAGTAGGGGTTTATAAT‐3′ and biFBXW7 reverse 5′‐CCAAAAACCATTTTTATAAAAAACAAT‐3′. The PCR products were ligated into plasmids using a StrataClone PCR Cloning Kit (Stratagene, La Jolla, CA, USA), which were then transformed into competent bacteria. More than 10 individual clones were isolated and amplified with the Illustra Templiphi Amplification Kit (GE Healthcare). Templiphi products were sequenced using a Big‐Dye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems) and an ABI3130 Genetic sequencer (Applied Biosystems). At least 10 clones from each parental allele were sequenced. Sequence data were analyzed using the QUMA quantification tool (http://quma.cdb.riken.jp/) for methylation analysis.
Cell lines
The human ovarian cancer cell line SHIN‐3 was purchased from Scienstaff (Nara, Japan). OVISE was purchased from JCRB Cell Bank (Osaka, Japan). The cells were grown in RPMI 1640 medium (Sigma, St. Louis, MO, USA) supplemented with 10% FBS GOLD (PAA, Pasching, Austria) with 100 units/mL penicillin and 100 μg/mL streptomycin (Gibco, Palo Alto, CA, USA) in 5% CO2 at 37°C.
Plasmids
pCMV‐Neo‐Bam p53 wt and pCMV‐Neo‐Bam p53 R175H were gifts from Bert Vogelstein (Addgene plasmid # 16434).27 These plasmids were introduced into SHIN‐3 and OVISE cells with the use of Lipofectamine 2000 transfection reagents (Invitrogen, Carlsbad, CA, USA).
Protein extraction and Western blotting
The cells were washed in ice‐cold PBS and lysed using CelLytic M (Sigma) following the manufacturer's instructions. Total protein (30 μg) was electrophoresed on 10% SDS‐polyacrylamide gels and transferred to nitrocellulose membranes (Immobilon; Merck Millipore, Darmstadt, Germany). The membranes were blocked and incubated overnight at 4°C with primary antibodies targeting p53 (FL‐393, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β‐actin (#4967, 1:1000; Cell Signaling, Danvers, MA, USA). After washing, membranes were incubated for 1 h with anti‐rabbit antibody (Sigma) diluted 1:4000. Specific protein bands were detected using the SuperSignal West Pico/Dura Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
For analysis of differences in two or more groups, Student's t‐test, χ2 analysis and anova were used. When the results of anova were significant, the Tukey–Kramer method was used. Overall survival curves were plotted according to the Kaplan–Meier method, with Wilcoxon analysis applied for comparison. Survival was measured from the day of the surgery. All differences were accepted as statistically significant at the level of P < 0.05. Statistical analysis was performed using JMP 9.0.2 (SAS Institute, Tokyo, Japan). Each P‐value in the figure is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Results
FBXW7 mutations were rare in ovarian cancer
According to previous reports, FBXW7α is ubiquitously expressed, whereas FBXW7β is expressed in the brain, and FBXW7γ is expressed in muscles.16 Therefore, we focused on FBXW7α expression and mutational status in this study. First, we examined FBXW7 and p53 mutations in clinical samples of 57 ovarian cancer patients (26 serous carcinomas, 11 mucinous carcinomas, 10 endometrioid carcinomas and 10 clear cell carcinomas). The results are shown in Figure 1 and Table S2. We only detected a single (silent) mutation in FBXW7. This result was consistent with two previous reports demonstrating that FBXW7 mutations are rare in ovarian cancer.14, 15 In contrast, p53 mutations were detected in 31.6% of the samples (18/57). In particular, serous carcinomas showed a high frequency of p53 mutations (16/26, 61.5%). It has been well established that p53 mutations are frequent in high‐grade serous carcinoma.28, 29
Figure 1.
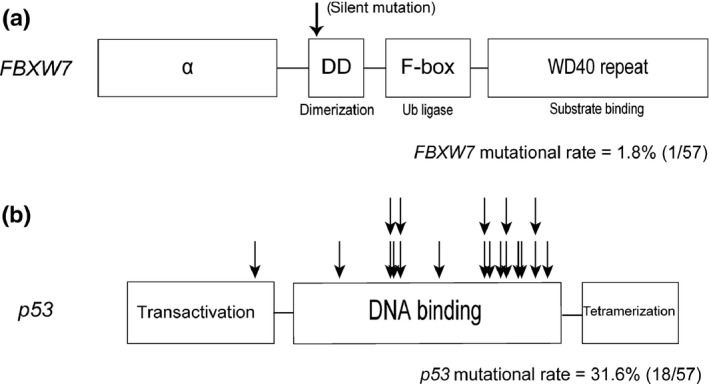
FBXW7 and p53 mutations in 57 ovarian cancer clinical samples. Arrows designate points of mutation. (a) Structure and the observed mutation in the FBXW7 gene in ovarian cancer patients. (b) Structure and the observed mutations in the p53 gene in ovarian cancer patients.
FBXW7 gene expression was low in serous carcinoma
The results of mutational analyses demonstrated that mutations in FBXW7 did not contribute to the malignant potential of ovarian cancer. Therefore, we next examined FBXW7 expression levels in 126 epithelial ovarian tumors (see Materials and Methods for pathological classification). The results are shown in Figure 2. FBXW7 gene expression levels in ovarian cancer samples were significantly lower than those in borderline and benign tumors (P < 0.01; Fig. 2a), indicating that FBXW7 expression levels were negatively correlated with malignant potential. Next, we examined FBXW7 expression in different histological subtypes of ovarian cancer. FBXW7 expression was the lowest in serous carcinomas, and increased in the following order: endometrioid carcinomas, clear cell carcinomas and mucinous carcinomas. In comparison to the average FBXW7 gene expression levels of benign tumors, those in endometrioid carcinomas and serous carcinomas were significantly reduced (P < 0.01 and P < 0.001, respectively). Moreover, the expression levels of FBXW7 in serous carcinomas were significantly lower than those in mucinous carcinomas and clear cell carcinomas (P < 0.05; Fig. 2b). In serous ovarian tumors, FBXW7 expression in serous carcinomas was significantly lower than that in serous cystadenomas (P < 0.01; Fig. S1a). However, FBXW7 expression levels in mucinous ovarian tumors did not significantly differ among benign, borderline and cancerous tumors (Fig. S1b). We could not compare the expression of FBXW7 of benign, borderline and cancerous tumors in clear cell and endometrioid tumors because clear cell and endometrioid benign/borderline tumors were rare.
Figure 2.
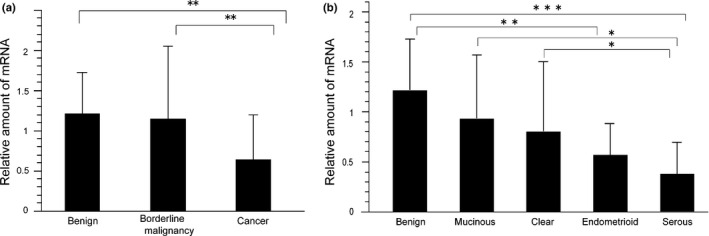
FBXW7 expression levels in 126 epithelial ovarian tumors. The data show the mean ± SD of values. P‐values in the figures are indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001. (a) FBXW7 mRNA expression levels in 126 epithelial ovarian tumors. (b) FBXW7 mRNA expression levels in 17 epithelial ovarian benign tumors, 15 mucinous carcinomas, 25 clear cell carcinomas, 17 endometrioid carcinomas and 26 serous carcinomas.
We also analyzed the clinicopathological features of ovarian cancer patients according to FBXW7 expression (Table 1). We divided cancer patients into two groups (high or low expression) according to FBXW7 expression levels. The high and low expression groups were defined according to an arbitrary value (i.e. patients were classified into the high expression group if they had higher FBXW7 expression than this value and into the low expression group if they had lower FBXW7 expression than this value). A total of 30 patients were included in the high expression group and 53 patients were included in the low expression group. Among clinicopathological features (menstruation status, clinical stage, lymph node metastasis, and histology) only histological subtype was significantly associated with FBXW7 expression. That is to say, the FBXW7 expression level in serous carcinoma was significantly suppressed. We thought that it might be due to the high frequency of p53 mutations in serous carcinoma because p53 influenced FBXW7 expression, as described later.
Table 1.
Clinicopathological features of ovarian cancer patients
| High expression (n = 30) | Low expression (n = 53) | P‐value | |
|---|---|---|---|
| Age (mean ± SD) | 55.1 ± 10.6 | 55.5 ± 15.2 | 0.5538 |
| Menstruation | |||
| Premenopause | 13 | 16 | 0.4486 |
| Postmenopouse | 14 | 32 | |
| Unknown | 3 | 5 | |
| Clinical stage | |||
| I | 22 | 28 | 0.0536 |
| II, III, IV | 8 | 25 | |
| Lymph node metastasis | |||
| (+) | 4 | 13 | 0.1486 |
| (−) | 23 | 39 | |
| NX | 3 | 1 | |
| Histology | |||
| Serous carcinoma | 2 | 24 | 0.0005*** |
| Clear cellcarcinoma | 11 | 14 | |
| Endometrioid carcinoma | 7 | 10 | |
| Mucinous carcinoma | 10 | 5 | |
The criterion for validating high or low FBXW7 expression groups was determined as greater or less than an arbitrary value.
FBXW7 expression in the mutant p53 group was significantly lower than that in the wild‐type p53 group
Given that Fbxw7 is a p53‐dependent tumor‐suppressor gene,7 we examined the correlation between FBXW7 expression levels and p53 mutation status. A total of 57 ovarian cancer samples were divided according to p53 status, and FBXW7 expression levels were analyzed (Fig. 3). All samples with p53 mutations were included in the low FBXW7 expression group, and the FBXW7 expression level in the p53 mutation group was significantly lower than that in the p53 wild‐type group. However, there was no significant difference in overall survival between the two groups (Fig. S2).
Figure 3.
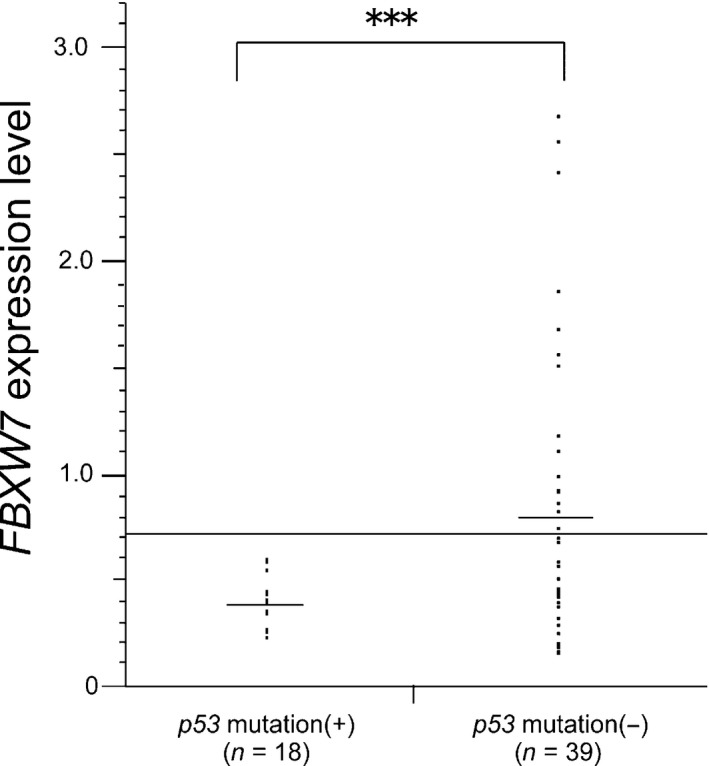
Dot plot of FBXW7 mRNA expression levels in ovarian cancer according to p53 status. The transverse line represents the baseline value of Fbxw7 expression dividing high and low expression groups. P‐values are indicated (***P < 0.001).
p53 mutations suppressed FBXW7 expression by hypermethylation of the 5′‐upstream region of FBXW7
We found that the FBXW7 expression levels in p53‐mutated ovarian cancer samples were significantly lower than those in p53 wild‐type samples (Fig. 3). To identify the underlying mechanisms how p53 alterations influenced FBXW7 expression levels, we investigated DNA methylation status of 5′‐upstream regions of FBXW7, because promoter hypermethylation of tumor‐suppressor genes is one of the important causes of oncogenesis.30, 31
To this end, we screened DNA methylation status of six samples using Infinium HumanMethylation450 BeadsChips. Among 25 probes surrounding the FBXW7 gene, two probes at chr4, 153437913 (hg19) and 153454027 (hg19), both located in the 5′‐upstream regions of FBXW7 gene (Fig. 4a), were hypomethylated in the FBXW7 high expression group and hypermethylated in the FBXW7 low expression group. According to these results, we designed a primer set around the probe 153437913 (hg19) (see the Materials and Methods for the primer sequence) for bisulfite PCR sequencing experiments, and analyzed 21 ovarian cancer samples (13‐p53 wild‐type samples and 8‐p53‐mutated samples). As shown in Figure 4(b,c), bisulfite PCR sequencing revealed that this region was significantly hypermethylated in p53‐mutated samples compared with that in p53 wild‐type samples (P = 0.006). These data suggested that p53 mutations suppressed FBXW7 expression by hypermethylation of FBXW7 5′‐upstream region in ovarian cancer.
Figure 4.
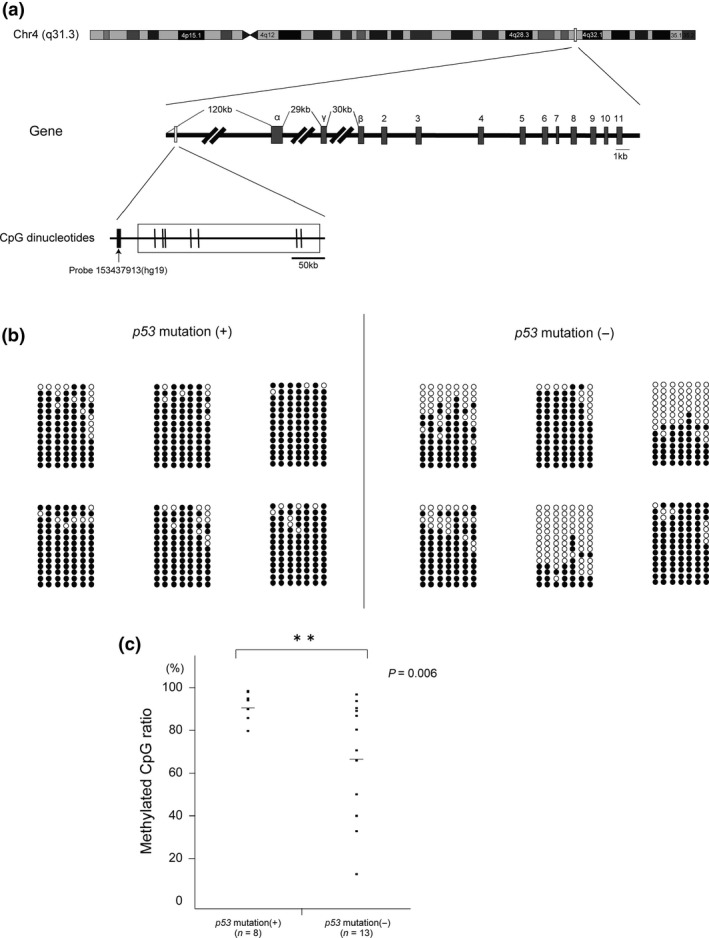
Hypermethylation of the 5′‐upstream regions of FBXW7 in p53‐mutated samples. The methylation status of seven CpG sites around the probe 153437913(hg19) was analyzed by bisulfite sequencing in 21 ovarian cancer samples (i.e. 8 p53‐mutated samples and 13 p53‐wild type samples). (a) Schematic map around the probe 153437913(hg19). Vertical short lines represent CpG sites. (b) Bisulfite sequencing profiles of representative 12 samples. The circles correspond to CpG sites denoted by thin bars in Figure 5(a). Closed circles represent methylated CpG and open circles represent unmethylated CpG. (c) Dot plot of methylated CpG ratio of the 5′‐upstream regions of FBXW7 according to p53 status.
Mutated p53 suppressed FBXW7 expression in ovarian cancer cell
Finally, we examined whether forced expression of mutated p53 would affect FBXW7 expression level in ovarian cancer cells. We introduced wild‐type p53 or mutated p53 (R175H) into two types of cell lines, OVISE (with wild‐type p53 and wild‐type FBXW7) and SHIN‐3 (with mutated p53 and wild‐type FBXW7), and then carried out quantitative RT‐PCR of FBXW7 (Fig. 5). FBXW7 expression was significantly suppressed by overexpression of p53 (R175H) compared with wild‐type p53 overexpression in OVISE, whereas FBXW7 expression was not suppressed by overexpression of p53 (R175H) in SHIN‐3, in which cells p53 was already mutated.
Figure 5.
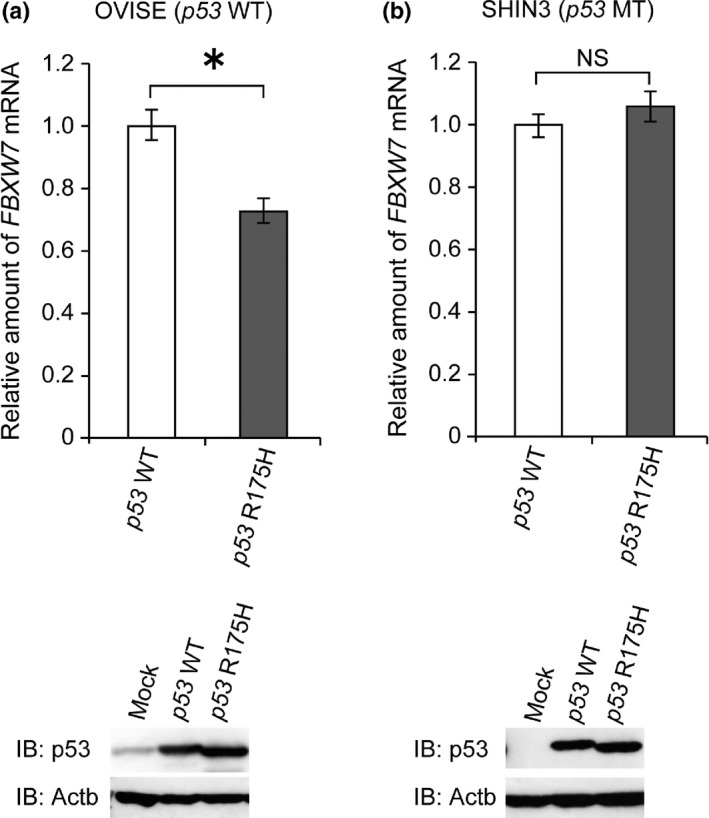
Mutated p53 suppressed FBXW7 expression in ovarian cancer cell. (a) Wild‐type p53 or mutated p53 (R175H) was introduced in OVISE (wild‐type p53 and FBXW7). FBXW7 mRNA expression level was suppressed by overexpression of mutated p53 compared with wild‐type p53 overexpression. The data shows means ± SD. The experiments were carried out three times. P‐values are indicated (*P < 0.05). (b) Wild‐type or mutated p53 (R175H) was introduced in SHIN‐3 (mutated p53 and wild‐type FBXW7). FBXW7 mRNA expression level was not affected by overexpression of wild‐type or mutated p53. The data shows means ± SD. The experiments were carried out three times.
Discussion
In the present study, we found that the mRNA level of FBXW7 was significantly suppressed in ovarian cancer compared with benign and borderline tumors. FBXW7 gene expression varied according to the cancer subtype. Among the four major histopathological subtypes (serous, mucinous, endometrioid and clear cell carcinoma), the greatest decrease was observed in serous carcinoma. FBXW7 was downregulated and the 5′‐upstream regions of FBXW7 was hypermethylated in p53 mutational group. In ovarian cancer cells, overexpression of mutated p53 (R175H) suppressed FBXW7 expression.
Functional loss of FBXW7 has been reported in many human cancers.11, 12, 13, 19, 20, 21, 22 Two mechanisms suppressing FBXW7 function are known: FBXW7 mutations and transcriptional suppression due to loss of p53. First, we examined FBXW7 mutations because such mutations have been detected in many human malignancies. Moreover, whole exome sequence approaches recently identified FBXW7 mutations in head and neck squamous cell carcinoma and uterine serous carcinoma.32, 33, 34 However, in the present study, we detected only one silent FBXW7 mutation in the ovarian cancer tissues. This result is consistent with those of previous reports demonstrating that FBXW7 mutations are infrequent in ovarian cancer.14, 15
Next we examined mRNA levels in ovarian cancer tissues, and found that FBXW7 gene expression was significantly suppressed in ovarian cancer specimens compared with borderline and benign ovarian tumors. These data indicated that FBXW7 expression was negatively correlated with the malignant potential of ovarian tumors. When we analyzed the FBXW7 expression data in light of p53 status, all p53 mutations were found in the low FBXW7 expression group. Consistent with this result, FBXW7 expression was significantly suppressed in the presence of p53 mutations. These data are consistent with the previous report that FBXW7 expression levels were low in most gastric cancer samples with p53 mutations.19 These results strongly support the notion that p53 regulates the expression of FBXW7.
Among the four major subtypes of ovarian cancers FBXW7 expression was most suppressed in serous carcinomas. It is widely known that each histological subtype is associated with distinct morphologic and molecular genetic alterations. High‐grade serous carcinomas are considered to arise from the epithelium of the distal fallopian tube and/or ovary with p53 mutations and dysfunction of BRCA1 and BRCA2.28, 29 In this report, we used the term “serous carcinoma” to indicate high‐grade serous carcinoma. High‐grade serous carcinomas are thought to represent de novo cancer, while mucinous carcinomas arise via an adenoma‐borderline tumor‐carcinoma sequence. The differences in the molecular events occurring during oncogenesis might be related to the distinct FBXW7 expression levels in these different types of ovarian cancers. In the present study, the p53 mutation rate was high (61.5%) in serous carcinomas. As mentioned above, p53 status was correlated with FBXW7 expression, and this was thought to be one reason why the expression of FBXW7 was low in serous carcinomas.
To investigate in detail the correlation between p53 status and FBXW7 expression, we focused on the DNA methylation status of the FBXW7 promoter region. It is well known that DNA hypermethylation of the promoter region of tumor suppressor genes is one of the epigenetic characteristics of cancer. We performed DNA methylation array and bisulfite PCR sequencing experiments and showed that the methylation status of the 5′‐upstream regions of FBXW7 was associated with the p53 status. These data suggested that FBXW7 expression would be also suppressed by promoter hypermethylation, like other tumor suppressor genes such as CDKN2A and MLH1, although further studies with more samples are required. p53 mutations were previously reported to be associated with DNMT1 protein overexpression, and DNMT1 overexpression is involved in hypermethylation of multiple tumor suppressor genes.35, 36 Our data suggested that FBXW7 might be one of such tumor suppressor genes transcriptionally suppressed by p53 mutations.
In conclusion, we demonstrated that dysfunctions of FBXW7 conferred malignant potential to ovarian tumors. This did not occur as a result of FBXW7 mutation but as a consequence of transcriptional downregulation. We suggest that FBXW7 expression would be suppressed by hypermethylation of FBXW7 5′‐upstream regions, which might be induced by p53 mutations. While chemotherapy is very useful in ovarian cancer treatment, FBXW7 dysfunctions have recently been reported to correlate with drug resistance.37, 38, 39 Moreover, FBXW7 degrades oncoproteins such as c‐Myc, cyclin E, Aurora A and Notch, all of which are involved in carcinogenesis, proliferation and metastases. However, the key substrates of FBXW7 that play the most important role in ovarian cancer development and its mechanisms remain unclear. Further studies are necessary to elucidate the details of these processes.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting information
Fig. S1. FBXW7 expression levels in serous and mucinous ovarian tumors.
Fig. S2. Kaplan–Meier overall survival curves of ovarian cancer patients based on FBXW7 expression levels.
Table S1. PCR primers for FBXW7/p53 mutation analysis.
Table S2. p53 mutation points in clinical samples from ovarian cancer patients.
Acknowledgments
We thank Chiharu Tayama, Naoko Sugahara and Hiromi Kamura (Department of Maternal‐Fetal Biology, National Research Institute for Child Health and Development), Tomoko Miyata (Department of Obstetrics and Gynecology, School of Medical Sciences, Kyushu University) and Dr Izumi Yamada (Department of Obstetrics and Gynecology, Juntendo University) for technical assistance. We appreciate the technical support from the Research Support Center, Graduate School of Medical Sciences, Kyushu University. This work was supported by grants‐in aid (21791560, 23390392 and 24592520) from the Ministry of Education, Culture, Sports, Science and Technology, Japan and the Health and Labour Sciences Research Grant for Research on Rare and Intractable Diseases (Jitsuyoka(Nanbyo)‐Ippan‐003).
Cancer Sci 107 (2016) 1399–1405
Funding Information
Ministry of Education, Culture, Sports, Science and Technology (21791560, 23390392, 24592520), Japan and the Health and Labour Sciences Research Grant for Research on Rare and Intractable Diseases (Jitsuyoka(Nanbyo)‐Ippan‐003).
References
- 1. Jemal A, Bray F, Center MM et al Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Ministry of Health, Labour, and Welfare . Mortality by sex in the Vital Statistics of Japan yearly survey in 2011 (for every 100,000 people). Tokyo: Ministry of Health, Labour, and Welfare, 2016. [Cited 03 Feb 2016]. Available from URL: http://www.mhlw.go.jp/. [Google Scholar]
- 3. Welcker M, Clurman BM. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 2007; 8: 83–93. [DOI] [PubMed] [Google Scholar]
- 4. Fujii Y, Yada M, Nishiyama M et al Fbxw7 contributes to tumor suppression by targeting multiple proteins for ubiquitin‐dependent degradation. Cancer Sci 2006; 97: 729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Onoyama I, Nakayama KI. Fbxw7 in cell cycle exit and stem cell maintenance. Cell Cycle 2008; 7: 3307–13. [DOI] [PubMed] [Google Scholar]
- 6. Wang Z, Inuzuka H, Zhong J et al Tumor suppressor functions of FBXW7 in cancer development and progression. FEBS Lett 2012; 586: 1409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao JH, Perez‐losada J, Wu D et al Fbxw7/cdc4 is a p53‐dependent haploinsufficient tumour suppressor gene. Nature 2004; 432: 775–9. [DOI] [PubMed] [Google Scholar]
- 8. Onoyama I, Tsunematsu R, Matsumoto A et al Conditional inactivation of Fbxw7 impairs cell‐cycle exit during T cell differentiation and results in lymphomatogenesis. J Exp Med 2007; 204: 2875–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsuoka S, Oike Y, Onoyama I et al Fbxw7 acts as a critical fail‐safe against premature loss of hematopoietic stem cells and development of T‐ALL. Genes Dev 2008; 22: 986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grim JE, Knoblaugh SE, Guthrie KA et al Fbw7 and p53 cooperatively suppress advanced and chromosomally unstable intestinal cancer. Mol Cell Biol 2012; 32: 2160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akhoondi S, Sun D, von der Lher N et al FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res 2007; 67: 9006–12. [DOI] [PubMed] [Google Scholar]
- 12. Spruck CH, Strohmaier H, Sangfelt O et al hCDC4 gene mutations in endometrial cancer. Cancer Res 2002; 62: 4535–9. [PubMed] [Google Scholar]
- 13. Malyukova A, Dohda T, von der Lehr N et al The tumor suppressor gene hCDC4 is frequently mutated in human T‐cell acute lymphoblastic leukemia with functional consequences for Notch signaling. Cancer Res 2007; 67: 5611–6. [DOI] [PubMed] [Google Scholar]
- 14. Kwak EL, Moberg KH, Wahrer DC et al Inflequent mutations of Archipelago (hAGO, hCDC4, Fbw7) in primary ovarian cancer. Gynecol Oncol 2005; 98: 124–8. [DOI] [PubMed] [Google Scholar]
- 15. Sgambato A, Cittadini A, Masciullo V et al Low frequency of hCDC4 mutations in human primary ovarian cancer. Gynecol Oncol 2007; 10: 553–5. [DOI] [PubMed] [Google Scholar]
- 16. Welcker M, Orian A, Grim JE, Eisenman RN, Clurman BE. A nucleolar isoform of the Fbw7 ubiquitin ligase regulates c‐Myc and cell size. Curr Biol 2004; 14: 1852–7. [DOI] [PubMed] [Google Scholar]
- 17. Matsumoto A, Onoyama I, Nakayama KI. Expression of mouse Fbxw7 isoforms is regulated in a cell cycle‐ or p53‐ dependent manner. Biochem Biophys Res Commun 2006; 350: 114–9. [DOI] [PubMed] [Google Scholar]
- 18. Kimura T, Gotoh M, Nakamura Y, Arakawa H. hCDC4b, a regulator of cyclin E, as a direct transcriptional target of p53. Cancer Sci 2003; 94: 431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yokobori T, Mimori K, Iwatsuki M et al p53‐altered FBXW7 expression determines poor prognosis in gastric cancer cases. Cancer Res 2009; 69: 3788–94. [DOI] [PubMed] [Google Scholar]
- 20. Iwatsuki M, Mimori K, Ishii H et al Loss of FBXW7, a cell cycle regulating gene, in colorectal cancer: clinical significance. Int J Cancer 2010; 126: 1828–37. [DOI] [PubMed] [Google Scholar]
- 21. Ibusuki M, Yamamoto Y, Shinriki S, Ando Y, Iwase H. Reduced expression of ubiquitin ligase FBXW7 mRNA is associated with poor prognosis in breast cancer patients. Cancer Sci 2011; 102: 439–45. [DOI] [PubMed] [Google Scholar]
- 22. Hagedorn M, Delugin M, Abraldes I et al FBXW7/hCDC4 controls glioma cell proliferation in vitro and is a prognostic marker for survival in glioblastoma patients. Cell Div 2007; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akhoondi S, Lindstrom L, Widschwendter M et al Inactivation of FBXW7/hCDC4‐β expression by promoter hypermethylation is associated with favorable prognosis in primary breast cancer. Breast Cancer Res 2010; 12: R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jahid S, Sun J, Edwards RA et al miR‐23a promotes the transition from indolent to invasive colorectal cancer. Cancer Discov 2012; 2: 540–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurashige J, Watanabe M, Iwatsuki M et al Overexpression of microRNA‐223 regulates the ubiquitin ligase FBXW7 in oesophageal squamous cell carcinoma. Br J Cancer 2012; 106: 182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Guo Y, Liang X et al MicroRNA‐223 functions as an oncogene in human gastric cancer by targeting FBXW7/hCdc4. J Cancer Res Clin Oncol 2012; 138: 763–74. [DOI] [PubMed] [Google Scholar]
- 27. Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild‐type p53. Science 1990; 249: 912–5. [DOI] [PubMed] [Google Scholar]
- 28. Prat J. New insights into ovarian cancer pathology. Ann Oncol 2012; 23: x111–7. [DOI] [PubMed] [Google Scholar]
- 29. Koshiyama M, Matsumura N, Konishi I. Recent concepts of ovarian carcinogenesis: type I and type II. Biomed Res Int 2014; 2014: 934261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones PA. DNA methylation and cancer. Oncogene 2002; 21: 5358–60. [DOI] [PubMed] [Google Scholar]
- 31. Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol 2005; 2: S4–11. [DOI] [PubMed] [Google Scholar]
- 32. Agrawal N, Frederick MJ, Pickering CR et al Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011; 333: 1154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Gallo M, O'Hara AJ, Rudd ML et al Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin‐remodeling and ubiquitin ligase complex genes. Nat Genet 2012; 44: 1310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao S, Choi M, Overton JD et al Landscape of somatic single‐nucleotide and copy‐number mutations in uterine serous carcinoma. Proc Natl Acad Sci USA 2013; 110: 2916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin RK, Wu CY, Chang JW et al Dysregulation of p53/Sp1 control leads to DNA methyltransferase‐1 overexpression in lung cancer. Cancer Res 2010; 70: 5807–17. [DOI] [PubMed] [Google Scholar]
- 36. Lin EK, Wang YC. Dysregulated transcriptional and post‐translational control of DNA methyltransferases in cancer. Cell Biosci 2014; 4: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Inuzuka H, Shaik S, Onoyama I et al SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 2011; 471: 104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wertz IE, Kusam S, Lam C et al Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 2011; 471: 110–4. [DOI] [PubMed] [Google Scholar]
- 39. Wang Z, Fukushima H, Gao D et al The two faces of FBW7 in cancer drug resistance. BioEssays 2011; 33: 851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. FBXW7 expression levels in serous and mucinous ovarian tumors.
Fig. S2. Kaplan–Meier overall survival curves of ovarian cancer patients based on FBXW7 expression levels.
Table S1. PCR primers for FBXW7/p53 mutation analysis.
Table S2. p53 mutation points in clinical samples from ovarian cancer patients.


