Summary
Renal impairment (RI) is a major complication of multiple myeloma (MM). This study aimed to characterize the single‐dose pharmacokinetics (PK) of the oral proteasome inhibitor, ixazomib, in cancer patients with normal renal function [creatinine clearance (CrCl) ≥90 ml/min; n = 20), severe RI (CrCl <30 ml/min; n = 14), or end‐stage renal disease requiring haemodialysis (ESRD; n = 7). PK and adverse events (AEs) were assessed after a single 3 mg dose of ixazomib. Ixazomib was highly bound to plasma proteins (~99%) in all renal function groups. Unbound and total systemic exposures of ixazomib were 38% and 39% higher, respectively, in severe RI/ESRD patients versus patients with normal renal function. Total ixazomib concentrations were similar in pre‐ and post‐dialyser samples collected from ESRD patients; therefore, ixazomib can be administered without regard to haemodialysis timing. Except for anaemia, the incidence of the most common AEs was generally similar across groups, but grade 3 and 4 AEs were more frequent in the severe RI/ESRD groups versus the normal group (79%/57% vs. 45%), as were serious AEs (43%/43% vs. 15%). The PK and safety results support a reduced ixazomib dose of 3 mg in patients with severe RI/ESRD.
Keywords: ixazomib, multiple myeloma, renal impairment, pharmacokinetics, dialysis
The prognosis of patients with multiple myeloma (MM) has improved since the introduction of novel agents including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs) and, more recently, monoclonal antibodies and histone deacetylase inhibitors (Kumar, 2010; Moreau, 2012; Mimura et al, 2015). Improved treatment outcomes have increased the focus on extended treatment, including maintenance therapy. However, the feasibility of long‐term treatment with current regimens is limited by toxicities and/or the need for regular clinic visits for parenteral treatment administration and/or monitoring. The development of novel agents with more convenient dosing and improved toxicity profiles would therefore represent a major advance in MM therapy.
The safety, tolerability, pharmacokinetics (PK), pharmacodynamics and clinical activity of the oral proteasome inhibitor ixazomib have been assessed previously in phase 1 and phase 1–3 studies in relapsed and/or refractory MM (RRMM) and newly diagnosed MM, relapsed or refractory systemic light‐chain amyloidosis (RRAL), lymphoma and solid tumours (Assouline et al, 2014; Kumar et al, 2014a,b; Merlini et al, 2014; Richardson et al, 2014; Gupta et al, 2015a; Moreau et al, 2015a; Smith et al, 2015). Among these cancer types, the efficacy of ixazomib has been particularly noted in MM. In November 2015, the United States Food and Drug Administration (US FDA) granted approval for the use of ixazomib in combination with lenalidomide and dexamethasone for the treatment of patients with MM who have received at least one prior therapy (http://www.ninlaro.com/downloads/prescribing-information.pdf), based on data from the TOURMALINE‐MM1 phase 3 trial in patients with RRMM (Moreau et al, 2015a).
Renal impairment (RI) is a frequent and severe complication of MM (Knudsen et al, 2000; Penfield, 2006). Depending on the threshold applied to define RI, up to half of patients with MM develop this condition (Clark et al, 1999; Tosi et al, 2015). MM‐associated RI presents distinct therapeutic challenges and, in its severe forms, is predictive of a poorer prognosis (Gonsalves et al, 2015; Khan et al, 2015; Laing et al, 2015). Previous studies suggest that the currently available PIs, bortezomib and carfilzomib, are effective and well tolerated in MM patients with varying degrees of RI (San Miguel et al, 2008; Chanan‐Khan et al, 2012; Badros et al, 2013). Accumulating evidence also suggests that bortezomib may even reverse RI or renal failure in MM (Dimopoulos et al, 2009a,b; Ludwig et al, 2010; Moreau et al, 2015b).
If the kidney plays an important role in the elimination of a drug, RI can result in increased drug exposure and may lead to increased toxicity. Changes in a drug's exposure may be particularly prominent in patients with severe RI and have been observed even when renal elimination is not the primary route of drug clearance (Sun et al, 2006; Nolin et al, 2008; Zhang et al, 2009). Accordingly, for most new drugs that are likely to be administered to patients with RI, including drugs that are not primarily excreted by the kidney, PK should be assessed in order to provide appropriate dosing recommendations based on guidance from the US FDA and European Medicines Agency (EMEA, 2004; US FDA, 2010).
Results from a pooled population PK analysis of cancer patients (with haematological and non‐haematological malignancies), which included four phase 1 trials of ixazomib, suggested that ixazomib clearance is not altered in patients with mild or moderate RI, defined as creatinine clearance (CrCl) 30–89 ml/min, supporting the conclusion that no ixazomib dose modification is needed in these patients (Gupta et al, 2015b). Based on these results, patients with mild or moderate RI have been included in the on‐going phase 3 clinical studies in patients with RRMM (NCT01564537), in patients with newly diagnosed MM (NCT01850524), as MM maintenance therapy following autologous stem cell transplantation (ASCT) (NCT02181413), as MM maintenance therapy after initial induction therapy without ASCT (NCT02312258) and in patients with RRAL (NCT01659658), and have received the same ixazomib dose (4 mg) as patients with normal renal function. However, the PK of ixazomib in patients with severe RI (CrCl <30 ml/min) or end‐stage renal disease (ESRD) requiring haemodialysis have not been studied previously. Therefore, the purpose of this study was to investigate the single‐dose PK of oral ixazomib in patients with RRMM or advanced solid tumours, and normal renal function, severe RI, or ESRD requiring haemodialysis, in order to provide posology recommendations in these patient populations.
Methods
Patients
Patients (aged ≥18 years) with MM diagnosed according to International Myeloma Working Group (IMWG) Criteria (International Myeloma Working Group, 2003) at initial diagnosis that had received ≥1 prior therapy were enrolled. Patients without measureable disease and those without disease progression (i.e. in stable disease) were also eligible for enrolment. Patients with a diagnosis of an advanced malignant solid tumour for whom standard, curative or life prolonging treatment did not exist or was no longer effective were also permitted.
An Eastern Co‐operative Oncology Group (ECOG) performance status of 0–2 was required for enrolment and all patients had an absolute neutrophil count ≥1·0 × 109/l and platelet count ≥75 × 109/l (>100 × 109/l for patients with solid tumours). Platelet transfusions to help patients meet eligibility criteria were not allowed within 3 d prior to ixazomib administration. Patients were required to have total bilirubin <1·5 times the upper limit of the normal range (ULN), and alanine aminotransferase and aspartate aminotransferase <3 times the ULN. Patients had to either be on haemodialysis (for the ESRD group) or have a calculated CrCl ≥90 ml/min or <30 ml/min (for the normal renal function or severe RI groups, respectively). CrCl was estimated using the Cockcroft–Gault equation (Cockcroft & Gault, 1976); blood sampling to determine spot serum creatinine for calculation of the CrCl consisted of at least two samples taken within 14 d of starting ixazomib treatment, with the most recent measurement performed within one week of starting treatment.
In Part A, higher than physiological dosing of dexamethasone (approximately 1 mg per d) or equivalent systemic steroid was also not permitted within 7 d before ixazomib administration. Patients with any co‐morbid systemic illness (including grade 1 peripheral neuropathy with pain or grade 2 or higher peripheral neuropathy) or other severe concurrent disease that, in the judgment of the investigator, would make the patient inappropriate for entry into the study or interfere significantly with the proper assessment of safety and toxicity of the prescribed regimen were not eligible. Systemic treatment with strong or moderate inhibitors of CYP1A2 or CYP3A, strong CYP3A inducers, or use of Ginkgo biloba or St. John's wort, within 14 d before ixazomib administration and during Part A of the study was also prohibited, as CYP3A and CYP1A2 were the primary contributors to the in vitro metabolism of ixazomib when examined at supra‐therapeutic concentrations (unpublished observations).
Study design
This phase 1/1b, open‐label, multicentre study was conducted in two parts (Fig 1).
Figure 1.
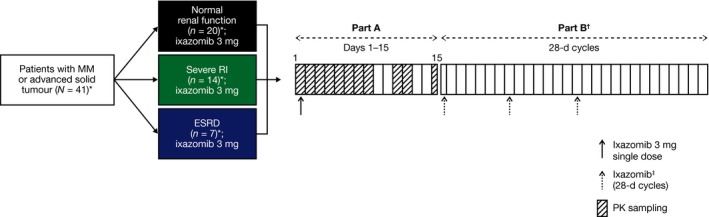
Study design overview. *Safety population. The PK‐evaluable population included all patients who received the protocol‐specified dose of ixazomib in Part A, did not receive any excluded concomitant medications through the completion of PK sampling, and had sufficient concentration–time data to permit reliable estimation of PK parameters by non‐compartmental analysis methods (normal renal function, n = 18, severe RI, n = 14, ESRD, n = 6); †Started on Day 15 after PK sample collection in Part A; ‡Patients received ixazomib at a dose of 4, 3, or 2·3 mg on Days 1, 8, and 15 of each 28‐d cycle in Part B, depending on the tolerability of treatment in Part A. MM, multiple myeloma; ESRD, end‐stage renal disease requiring haemodialysis; PK, pharmacokinetics; RI, renal impairment.
In Part A, all patients received a single 3 mg dose of ixazomib on day 1. Patients who tolerated ixazomib in Part A could choose to participate in Part B, which started following collection of the Day 15 PK sample of Part A. In Part B, patients received ixazomib at a dose of 4, 3 or 2·3 mg on days 1, 8, and 15 of each 28‐d cycle with the starting dose depending on the tolerance to treatment in Part A and subsequent dose modifications or interruptions as clinically indicated. Dexamethasone (40 mg, or 20 mg for patients aged >75 years) was administered to some patients with RRMM at the discretion of the investigator, on days 1, 8, 15 and 22 in Part B of the study.
Ixazomib was given on an empty stomach, at least one hour before or at least two hours after food (Gupta et al, 2016). Each dose of ixazomib was to be given orally with approximately 8 oz (240 ml) of water. Antiviral therapies, such as acyclovir, valacyclovir or other antivirals, were also required as clinically indicated.
The study was conducted at six sites in the US and Canada between 24 September 2013 (first patient enrolled) and 7 September 2015 (data cut‐off). The final protocol, amendments and informed consent documentation were reviewed and approved by the Institutional Review Board(s) and/or Independent Ethics Committee(s) at each of the participating centres. All patients provided written informed consent, and the trial was conducted according to the stipulations set out in the Declaration of Helsinki and International Conference on Harmonization Guideline for Good Clinical Practice. The study was registered at www.clinicaltrials.gov as NCT01830816.
Study objectives
The primary objective of the study was to characterize the single‐dose PK of ixazomib in cancer patients with normal renal function, severe RI or ESRD requiring haemodialysis. Secondary objectives were to characterize the safety and tolerability of oral ixazomib, and to determine the overall response rate (ORR) and duration of response (DOR) in patients with RRMM.
Assessments
To measure the ixazomib plasma concentrations, 3 ml blood PK samples were collected before dosing, and at multiple time points over 336 hours (15 d) after the single ixazomib dose in Part A. Blood samples were collected from patients prior to dosing, and 0·5, 1, 1·5, 2, 3, 4, 8, 24, 29 (only in patients with ESRD), 30 (only in patients with ESRD), 48, 72, 96, 120, 144, 168, 240, 264 and 336 h after the Day 1 dose. For patients with ESRD requiring haemodialysis, pre‐ and post‐dialyser plasma samples were also collected hourly during the first 4‐h dialysis session that occurred approximately 24–28 h after the Day 1 dose in Part A. Plasma concentrations of ixazomib were measured using a validated liquid chromatography/tandem mass spectrometry (LC‐MS/MS) assay with a dynamic range of 0·5 to 500 ng/ml.
An additional blood sample was collected before ixazomib administration on Day 1 of Part A for the in vitro estimation of ixazomib plasma protein binding. In vitro protein binding was assessed by rapid equilibrium dialysis. The protein binding assay was conducted in triplicate (sample volume permitting) using rapid equilibrium dialysis and the concentration of ixazomib in the plasma:buffer (50:50 v/v) samples was determined by LC‐MS/MS. Concentrations of ixazomib in dialysate fluid used in haemodialysis were not measured due to challenges associated with the instability of ixazomib in dialysate. All PK assessments were based on Part A of the study.
Adverse events (AEs), including serious AEs (SAEs), were evaluated throughout treatment and up to 30 d after the last dose of ixazomib, and graded using the National Cancer Institute's Common Terminology Criteria for AEs, version 4.03 (evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_8.5x11.pdf). Myeloma disease response was assessed by the individual investigators in accordance with IMWG uniform criteria (Durie et al, 2006). A single bone marrow assessment was performed to document complete responses (CRs). DOR was defined as the length of time between the date of first documented response (CR, very good partial response [VGPR] or partial response [PR]) to the date of first documentation of progressive disease for responders.
Statistical analysis
Ixazomib plasma PK parameters were calculated using non‐compartmental methods with Phoenix WinNonlin version 6.2 (Pharsight, St. Louis, MO, USA). The calculated parameters included the peak plasma concentration (C max) and the area under the concentration‐time curve from time 0 to the time of the last quantifiable concentration (AUC0‐last). The in vitro protein binding assay data were used to calculate the fraction unbound and the fraction bound for ixazomib. Unbound C max and AUC parameters were calculated using the experimentally determined fraction unbound values and the associated PK parameters. PK parameters were summarized using descriptive statistics.
For estimation of the effect of RI on the PK of ixazomib, a mixed effects analysis of variance (anova) on the natural log‐transformed, unbound PK parameters (C max and AUC0‐last) was performed with renal function group as a fixed effect. The normal renal function group was the reference group for calculating the ratios and 90% confidence intervals (CIs) of the ratio of geometric means. After log transformation, unbound C max and AUC0‐last were analysed separately. Point estimates and adjusted 90% CIs for the difference between renal function groups were calculated and exponentially back‐transformed to provide point and CI estimates for the ratios of interest. Patients in Part A who did not have adequate PK assessments or who did not follow the study design were not PK‐evaluable.
Haemodialysis clearance (CLHD) was calculated using the following equation: CLHD = QBIN × R × f. In this equation, QBIN is the blood flow rate into the dialyser, R is the plasma‐to‐blood ratio [estimated using the blood‐to‐plasma AUC ratio (12·7) following single‐dose administration], and f is the fraction of ixazomib removed during haemodialysis, calculated as (AUCin – AUCout)/AUCin, where AUCin and AUCout are the area under the concentration‐time curve during the haemodialysis interval using samples collected at the entry and exit of the dialyser, respectively (Chen et al, 2007; Khadzhynov et al, 2012; Dahlke et al, 2016).
The response‐evaluable population consisted of patients with RRMM who received at least 1 cycle of ixazomib treatment in Part B and had at least 1 post‐baseline response assessment. Patients who were enrolled while in remission from their prior therapy and had no measurable disease at the time of enrolment were excluded from the response‐evaluable population. The safety population included all patients who received ≥1 dose of ixazomib.
Results
Patients
As of the data cut‐off date (7 September 2015), 41 patients were enrolled in the study (20, 14 and 7 patients in the normal renal function, severe RI and ESRD groups, respectively); 61% were female, 66% Caucasian and 29% African American (Table 1). The median age was 61·0 years (range, 40–82) and the median weight was 74·7 kg (range, 46–147).
Table 1.
Overall patient demographics (safety populationa) and disease characteristics in patients with RRMM
| Characteristic | Normal renal function (N = 20) | Severe RI (N = 14) | ESRD (N = 7) | Combined Severe RI/ESRD (N = 21) | Total (N = 41b) |
|---|---|---|---|---|---|
| Median age, years (range) | 55·0 (42–70) | 74·5 (58–82) | 61·0 (40–82) | 68·0 (40–82) | 61·0 (40–82) |
| Median weight, kg (range) | 87·3 (62–147) | 54·3 (46–100) | 82·3 (48–128) | 61·7 (46–128) | 74·7 (46–147) |
| Male, n (%) | 10 (50) | 2 (14) | 4 (57) | 6 (29) | 16 (39) |
| Race, n (%) | |||||
| White | 13 (65) | 11 (79) | 3 (43) | 14 (67) | 27 (66) |
| Black or African American | 6 (30) | 3 (21) | 3 (43) | 6 (29) | 12 (29) |
| Asian | 0 | 0 | 1 (14) | 1 (5) | 1 (2) |
| Not reported | 1 (5) | 0 | 0 | 0 | 1 (2) |
| RRMM patients | N = 16 | N = 14 | N = 7 | N = 21 | N = 37 |
| Median time from initial diagnosis to first ixazomib dose, months (range) | 50·33 (19·5–153·1) | 41·15 (8·0–103·1) | 32·39 (11·5–83·2) | 38·01 (8·0–103·1) | 44·02 (8·0–153·1) |
| MM subtype, n (%) | |||||
| IgG | 10 (63) | 12 (86) | 3 (43) | 15 (71) | 25 (68) |
| IgA | 3 (19) | 2 (14) | 2 (29) | 4 (19) | 7 (19) |
| IgM | 1 (6) | 1 (7) | 1 (14) | 2 (10) | 3 (8) |
| Free Kappa light chain | 2 (13) | 1 (7) | 1 (14) | 2 (10) | 4 (11) |
| Free Lambda light chain | 0 | 0 | 1 (14) | 1 (5) | 1 (3) |
| Not detected | 2 (13) | 0 | 1 (14) | 1 (5) | 3 (8) |
| ECOG PS, n (%) | |||||
| 0 | 8 (50) | 3 (21) | 2 (29) | 5 (24) | 13 (35) |
| 1 | 7 (44) | 11 (79) | 2 (29) | 13 (62) | 20 (54) |
| 2 | 1 (6) | 0 | 3 (43) | 3 (14) | 4 (11) |
| ISS stage at baseline, n (%) | |||||
| I | 6 (38) | 0 | 0 | 0 | 6 (16) |
| II | 5 (31) | 2 (14) | 0 | 2 (10) | 7 (19) |
| III | 0 | 9 (64) | 5 (71) | 14 (67) | 14 (38) |
| Unknown | 5 (31) | 3 (21) | 2 (29) | 5 (24) | 10 (27) |
| Prior anticancer therapy, n (%) | 16 (100) | 14 (100) | 7 (100) | 21 (100) | 37 (100) |
| Median lines of prior therapy | 2·5 | 2·5 | 4·0 | 3·0 | 3·0 |
| Type of prior therapy, n (%) | |||||
| Corticosteroid based | 16 (100) | 14 (100) | 7 (100) | 21 (100) | 37 (100) |
| Bortezomib based | 15 (94) | 14 (100) | 7 (100) | 21 (100) | 36 (97) |
| Carfilzomib based | 4 (25) | 5 (36) | 2 (29) | 7 (33) | 11 (30) |
| Thalidomide based | 6 (38) | 4 (29) | 6 (86) | 10 (48) | 16 (43) |
| Lenalidomide based | 14 (88) | 8 (57) | 6 (86) | 14 (67) | 28 (76) |
| Refractory to PI | 5 (31) | 5 (36) | 5 (71) | 10 (48) | 15 (41) |
| Refractory to IMiD | 5 (31) | 5 (36) | 3 (43) | 8 (38) | 13 (35) |
| Refractory to other treatments | 2 (12) | 3 (21) | 4 (57) | 7 (33) | 9 (24) |
ECOG PS, Eastern Cooperative Oncology Group performance status; ESRD, end‐stage renal disease requiring haemodialysis; Ig, immunoglobulin; IMiD, immunomodulatory drug; ISS, International Staging System; PI, proteasome inhibitor; RI, renal impairment; RRMM, relapsed and/or refractory multiple myeloma; SD, standard deviation.
All patients who received at least one dose of ixazomib.
37 patients had RRMM and 4 had solid tumours (2 colon, 1 liver, 1 thyroid).
Of the 41 patients, 37 patients had RRMM and 4 had solid tumours (2 colon, 1 liver, 1 thyroid). All patients (n = 41) had received systemic therapy prior to the study. The median number of prior lines of therapy for RRMM patients was 3 (range, 1–8). All RRMM patients had received corticosteroids as part of their prior therapy, most (n = 36; 97%) received therapy that contained bortezomib, and most (n = 30; 81%) received therapy containing an IMiD. In total, 41% of patients were refractory to a proteasome inhibitor (bortezomib and/or carfilzomib) and 35% were refractory to an IMiD. Among the four patients with solid tumours, one patient received sorafenib and oxaliplatin, one had radioactive iodine treatment and sorafenib, and two had bevacizumab‐based chemotherapy. All four patients with advanced solid tumours had Stage IV or IVB cancer and had normal renal function with CrCl ≥90 ml/min at baseline; for these patients, the median time from initial diagnosis to first ixazomib dose was 76·48 months (range, 17·8–134·8 months).
In total, 36 patients participated in Part B. Seven patients (17%) were receiving on‐going study treatment at the time of the data cut‐off (7 September 2015). Of these seven patients, four patients had normal renal function, three had severe RI and none had ESRD requiring haemodialysis. The reasons among the 29 patients who discontinued from Part B of the study were progressive disease (n = 22; 76%), AE (n = 6; 21%) and symptomatic deterioration (n = 1; 3%).
Treatment exposure
Among the 36 patients who participated in Part B, (normal renal function, 17; severe RI, 13; ESRD, 6), all patients in the normal renal function and severe RI groups, and four patients (67%) in the ESRD group received 4 mg of ixazomib at the start of Part B. Twenty‐nine patients received weekly dexamethasone in combination with ixazomib during Part B of the study, with 23 and six patients receiving 40 mg and 20 mg, respectively. Overall, as of the data cut‐off date, patients with normal renal function had been treated for a median of 6 cycles (range, 1–17), compared with 3 cycles (range, 1–17) in the severe RI group and 1·5 cycles (range, 1–5) in the ESRD group, with a mean relative dose intensity of 93·5%, 79·6% and 61·6% for patients with normal renal function, severe RI and ESRD, respectively.
Pharmacokinetics
Thirty‐eight patients (93%) had reportable PK parameters (C max or AUC) and were PK‐evaluable (18, 14 and 6 in the normal renal function, severe RI and ESRD groups, respectively) (Table 2). Fig 2 shows the mean plasma ixazomib concentrations over time by renal function group. Ixazomib was highly bound to plasma proteins with a similar mean fraction bound of approximately 99% in all three renal function groups. After a single 3 mg dose, ixazomib was rapidly absorbed in all three renal function groups, with a median T max of 1·04, 1·0 and 1·25 h in the normal renal function, severe RI and ESRD groups, respectively.
Table 2.
Summary of PK parameters of ixazomib (PK‐evaluable population)
| Parametera | Renal function group | ||
|---|---|---|---|
| Normal renal function (n = 18) | Severe RI (n = 14) | ESRD (n = 6) | |
| Total PK parameters | |||
| T max, hb | 1·04 (0·467–4) | 1·00 (0·45–1·5) | 1·25 (0·983–7) |
| C max, ng/ml | 25·8 (56) | 45·3 (81) | 18·7 (82) |
| AUC0–last, h·ng/ml | 575 (38)c | 813 (51)d | 783 (35) |
| Unbound PK parameters | |||
| C max, ng/ml | 0·300 (66) | 0·478 (86) | 0·213 (57) |
| AUC0–last, h·ng/ml | 6·64 (61)c | 9·25 (55)d | 8·93 (55) |
AUC0‐last, area under the concentration‐time curve from time 0 to the time of the last quantifiable concentration; C max, peak plasma concentration; ESRD, end‐stage renal disease requiring haemodialysis; PK, pharmacokinetics; RI, renal impairment; T max, the first observed time of C max.
Values shown are geometric mean (% coefficient of variation) unless otherwise specified.
Values are median (range).
n = 15.
n = 10.
Figure 2.
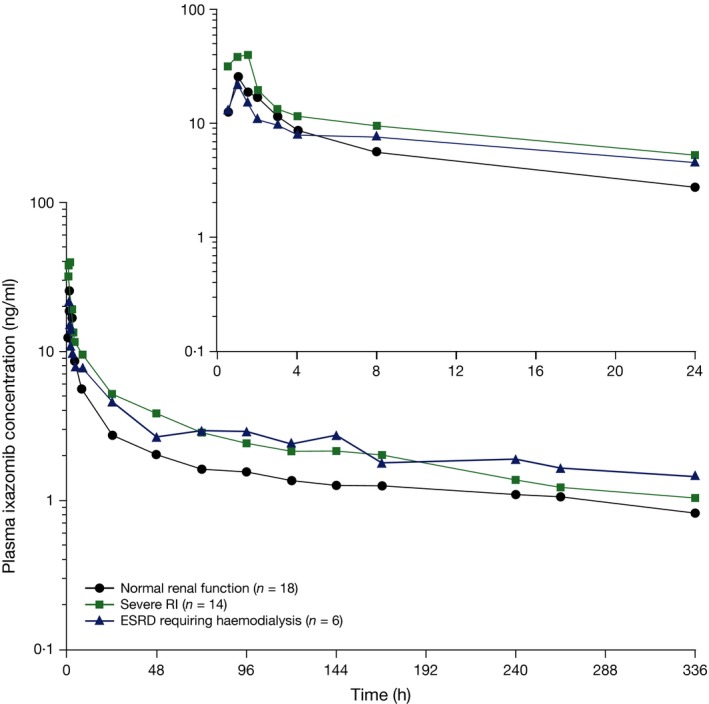
Mean plasma ixazomib concentration–time plots. Mean plasma ixazomib concentration‐time plots after oral administration of 3 mg ixazomib in Part A of the study in patients with normal renal function, severe RI and ESRD requiring haemodialysis (log‐linear scale). The inset shows the mean plasma ixazomib concentrations of the first 24 h post‐dose. ESRD, end stage renal disease requiring haemodialysis; RI, renal impairment.
Geometric mean [% coefficient of variation (CV)] unbound C max was 0·30 (66), 0·478 (86) and 0·213 (57) ng/ml, respectively, in the normal renal function, severe RI and ESRD groups. The corresponding values for unbound AUC0‐last were 6·64 (61), 9·25 (55) and 8·93 (55) h.ng/ml, respectively. A comparison of unbound AUC0‐last values in the normal renal function and severe RI/ESRD group is presented in Fig 3. The median value of unbound AUC in the combined severe RI/ESRD requiring haemodialysis group was greater than the 75th percentile of the corresponding distribution in the normal renal function group, suggesting a shift in the exposure distribution to higher values in the setting of severe RI or ESRD requiring haemodialysis, although a high level of interpatient variability was observed based on visual inspection of the box and whisker plots. As the geometric mean and observed distribution of total systemic exposures in the severe RI and ESRD groups appeared similar, a single statistical analysis of PK parameters using pooled data from the severe RI and ESRD groups in reference to the normal renal function group was performed. This analysis resulted in geometric least squares mean ratios (90% CI) for unbound C max and AUC0‐last of 1·25 (0·79–1·98) and 1·38 (0·93–2·04), respectively (Table 3). Similarly, geometric least squares mean ratios (90% CI) of 1·35 (0·86–2·10) and 1·39 (1·04–1·86), respectively, were calculated for total C max and AUC0‐last.
Figure 3.
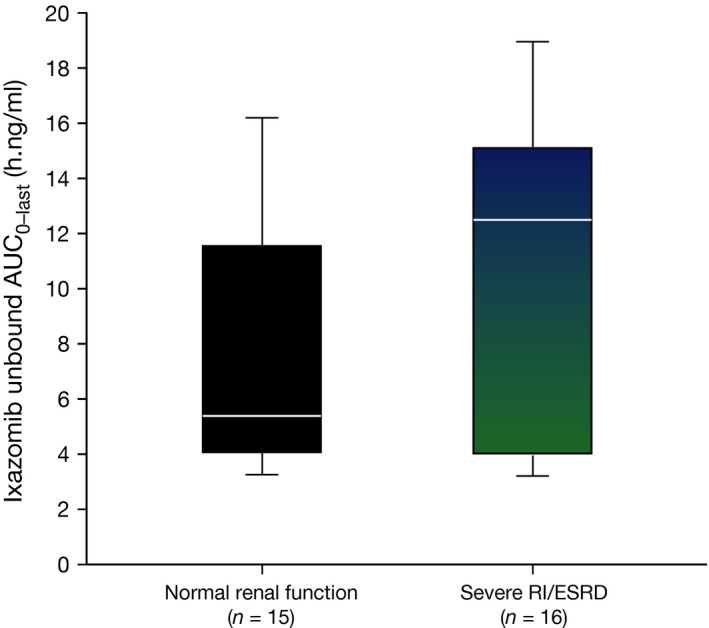
Comparison of unbound AUC 0–last values by renal function category. Ixazomib unbound AUC 0–last in the normal renal function and combined severe RI/ESRD groups. The box lines denote the 25th, 50th, and 75th percentile. Whiskers represent the 10th and 90th percentile for each renal function category. AUC 0‐last, area under the plasma ixazomib concentration‐time curve from time 0 to the time of the last quantifiable concentration; ESRD, end stage renal disease requiring haemodialysis; RI, renal impairment.
Table 3.
Geometric least squares mean ratios (90% CIs) for unbound C max and AUC0–last.
| Parameter | Geometric least‐squares mean ratio (90% CI) | ||
|---|---|---|---|
| Severe RI versus normal renal function | ESRD versus normal renal function | Combined RIa versus normal renal function | |
| Unbound C max, ng/ml | 1·60 (0·99–2·58) | 0·71 (0·38–1·34) | 1·25 (0·79–1·98) |
| Unbound AUC0–last, h.ng/ml | 1·39 (0·88–2·20) | 1·34 (0·78–2·31) | 1·38 (0·93–2·04) |
AUC0‐last, area under the concentration‐time curve from time 0 to the time of the last quantifiable concentration; CI, confidence interval; C max, peak plasma concentration; ESRD, end‐stage renal disease requiring haemodialysis; RI, renal impairment.
Patients with severe RI or ESRD requiring haemodialysis combined.
Ixazomib concentrations were similar in pre‐ and post‐dialyser samples collected hourly from patients with ESRD during the 4‐h haemodialysis session (Fig 4). In ESRD patients requiring haemodialysis, the haemodialysis ixazomib clearance was less than 2 ml/min in all patients.
Figure 4.
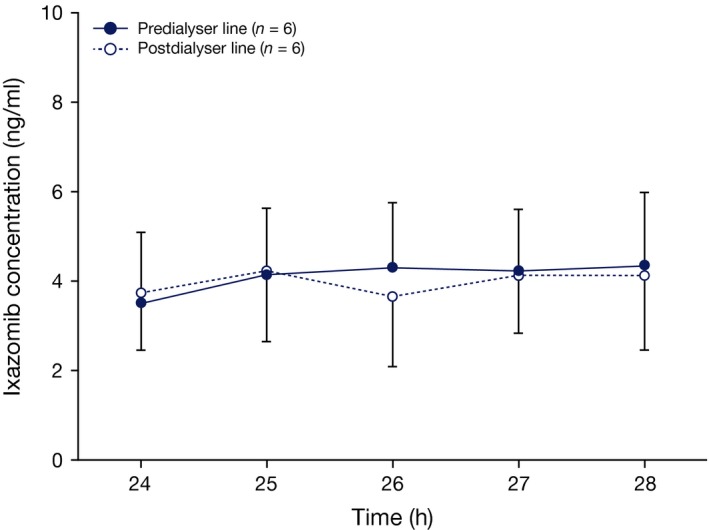
Ixazomib concentrations in pre‐ and post‐dialyser samples. Mean ixazomib concentrations in samples collected from the pre‐ and post‐dialyser lines during the haemodialysis session that occurred approximately 24–28 h after ixazomib administration in Part A of the study. Error bars indicate standard deviation.
Safety
All 41 patients received at least one dose of ixazomib and were included in the safety population (Table 4). In total, 95% of patients had a treatment‐emergent AE (TEAE) of any grade (reported from the first dose of ixazomib until 30 d after administration of the last dose of ixazomib in either Part A or Part B of the study; data cut‐off, 7 September 2015). The most common TEAEs were diarrhoea (39%), nausea (34%), vomiting (32%), fatigue (29%) and anaemia (22%). The incidence of these TEAEs was generally similar across renal function groups, except for the AE of anaemia, which was more common in patients in the severe RI and ESRD groups (5%, 43%, and 29% of patients in the normal renal function, severe RI, and ESRD groups). The most common grade ≥3 TEAEs were anaemia (15%), hypotension (7%) and decreased platelet count (7%). The incidences of grade ≥3 AEs were greater in the severe RI and ESRD groups versus patients with normal renal function (79% and 57% vs. 45%, respectively), as were the incidences of serious AEs (43% and 43% vs. 15%, respectively).
Table 4.
Overall summary of TEAEs and most common TEAEs occurring in ≥20% of all patients (safety population)
| AE, n (%) | Normal renal function (N = 20) | Severe RI (N = 14) | ESRD (N = 7) | Total (N = 41) |
|---|---|---|---|---|
| Any TEAE | 19 (95) | 14 (100) | 6 (86) | 39 (95) |
| Grade ≥3 | 9 (45) | 11 (79) | 4 (57) | 24 (59) |
| Study drug‐related TEAE | 16 (80) | 12 (86) | 4 (57) | 32 (78) |
| Grade ≥3 | 7 (35) | 7 (50) | 1 (14) | 15 (37) |
| SAE | 3 (15) | 6 (43) | 3 (43) | 12 (29) |
| Drug‐related SAE | 3 (15) | 2 (14) | 0 | 5 (12) |
| TEAE resulting in ixazomib discontinuation | 2 (10) | 4 (29) | 0 | 6 (15) |
| TEAE resulting in ixazomib dose reduction | 4 (20) | 4 (29) | 0 | 8 (20) |
| On‐study deathsa | 0 | 2 (14) | 1 (14) | 3 (7) |
| Most common TEAEs | ||||
| Diarrhoea | 9 (45) | 6 (43) | 1 (14) | 16 (39) |
| Nausea | 7 (35) | 5 (36) | 2 (29) | 14 (34) |
| Vomiting | 6 (30) | 5 (36) | 2 (29) | 13 (32) |
| Fatigue | 5 (25) | 6 (43) | 1 (14) | 12 (29) |
| Anaemia | 1 (5) | 6 (43) | 2 (29) | 9 (22) |
| Constipation | 5 (25) | 3 (21) | 1 (14) | 9 (22) |
| URT infection | 5 (25) | 1 (7) | 1 (14) | 7 (17) |
| Arthralgia | 3 (15) | 2 (14) | 1 (14) | 6 (15) |
| Dizziness | 1 (5) | 4 (29) | 1 (14) | 6 (15) |
| Dyspnoea | 2 (10) | 3 (21) | 1 (14) | 6 (15) |
| Peripheral oedema | 2 (10) | 4 (29) | 0 | 6 (15) |
| Hypotension | 1 (5) | 3 (21) | 1 (14) | 5 (12) |
AE, adverse event; ESRD, end‐stage renal disease requiring haemodialysis; SAE, serious adverse event; TEAE, treatment‐emergent adverse event; URT, upper respiratory tract.
Deaths that occurred between the first dose of study drug and 30 d of the last dose.
The number of AEs leading to ixazomib dose reductions (29% vs. 20%) and discontinuations (29% vs. 10%) was also greater in patients with severe RI versus the normal renal function group. There were no dose reductions for either ixazomib or dexamethasone in the ESRD group. The most common reasons for ixazomib dose reductions were increased weight (n = 2, one patient with medical history of being overweight, and one patient with arthritis and left leg weakness), decreased platelet count, and peripheral sensory neuropathy (two patients each). Six patients developed peripheral neuropathy (15%; grade 1, n = 3; grade 2, n = 2; grade 3, n = 1) and two patients discontinued due to peripheral neuropathies (one patient with normal renal function had grade 2 peripheral sensory neuropathy and one patient with severe RI had grade 1 peripheral neuropathy). There were three on‐study deaths (2 in the severe RI group and 1 in the ESRD group; all had RRMM); one patient in the severe RI group died of acute hypoxaemic respiratory failure that was considered related to study drug (acute respiratory distress syndrome from acute pancreatitis and possible pneumonia were considered as alternative aetiologies) and the other two patients died of AEs that were not considered related to study drug.
Overall, the most common study drug‐related TEAEs were diarrhoea (29%), nausea (29%) and vomiting (27%). There were no readily apparent trends of increased incidence among the most commonly reported study drug‐related TEAEs (≥20% of all patients) by renal function group. However, investigator‐attributed drug‐related anaemia occurred more frequently in the severe RI group than in the normal renal function group (29% vs. 5% of patients; 0% in ESRD patients), although this was not adjusted for time on study treatment.
Of the four patients with solid tumours, three (75%) experienced at least 1 TEAE. In two of the patients, these were grade 1 or 2 in severity and were not related to ixazomib treatment. The other patient had several grade 1 or 2 TEAEs, some of which (hypoglycaemia, hyperglycaemia and pruritis) were considered to be drug‐related, as well as one grade 3, ixazomib‐related acute renal failure and one grade 4 hypoglycaemia (not related to ixazomib).
Preliminary efficacy
In total, 25 patients with RRMM had measurable disease at baseline. Of these 25 patients, 18 were response‐evaluable (3 with normal renal function, 11 with severe RI and 4 with ESRD). The remaining seven patients had no baseline assessments or no post‐treatment assessments. The ORR (confirmed and unconfirmed, CR+PR [including VGPR]) was 28% (five of 18 evaluable patients): no patients in the normal renal function or ESRD groups had a response; five patients with severe RI had a PR, two of whom were refractory to prior PI therapy. Among the 5 severe RI patients who had a PR, DOR ranged from not available (censored just after the response assessment) to 225 d, with a median of 117 d (censored).
Discussion
Renal impairment is a major complication in patients with MM. Therefore, an understanding of the impact of RI on the PK of a drug used to treat MM is important to inform appropriate dosing, in order to ensure safe and effective pharmacotherapy. Several subanalyses and studies suggest that treatment with bortezomib‐based therapy is effective and well tolerated in MM patients with RI and can at least partially overcome the negative prognostic impact of RI in these patients (San Miguel et al, 2008; Dimopoulos et al, 2010; Ludwig et al, 2010; Morabito et al, 2011; Scheid et al, 2014). Carfilzomib has also demonstrated clinical efficacy in RRMM patients with varying degrees of RI (CrCl 50–80 ml/min, 30–49 ml/min, <30 ml/min and chronic haemodialysis) in a phase 2 study, and the safety and PK properties of carfilzomib did not appear to be influenced by the level of baseline RI (Badros et al, 2013). However, patients with CrCl <50 ml/min were not included in the subsequent phase 3 trial evaluating carfilzomib, lenalidomide and dexamethasone (Stewart et al, 2015).
The primary objective of this study was to characterize the single‐dose PK of ixazomib in cancer patients with normal renal function or severe RI, including ESRD requiring haemodialysis, to provide posology recommendations in these renally impaired patients. Following a single 3 mg dose, ixazomib was rapidly absorbed in all 3 renal function groups, with a median T max of 1·0–1·25 h. The geometric least squares mean unbound and total AUC ratios for the combined severe RI/ESRD group versus the normal renal function group were 1·38 and 1·39, respectively. Consequently, systemic exposures of ixazomib were 38% and 39% higher in patients with severe RI or ESRD requiring dialysis. Therefore, a reduced starting dose of 3 mg is recommended for MM patients with severe RI or ESRD requiring dialysis, because a 3 mg dose would be expected to provide systemic exposures (AUC) of ixazomib in these patients that are comparable to those observed in patients with normal renal function after receiving the recommended phase 3 dose of 4 mg.
Ixazomib was approximately 99% bound to plasma proteins in all three renal function categories, indicating RI does not alter the extent of plasma protein binding for ixazomib. In patients with ESRD requiring haemodialysis, ixazomib concentrations were similar in the pre‐ and post‐dialyser lines throughout the haemodialysis session, suggesting that ixazomib is not dialysable. In addition, haemodialysis ixazomib clearance was less than 2 ml/min in all patients with ESRD requiring haemodialysis, which represents less than 10% of overall systemic clearance (2 l/h) (Gupta et al, 2015b), and suggests that haemodialysis has a negligible impact on ixazomib clearance from the body. These findings are consistent with the high degree of ixazomib binding to plasma proteins and indicate that ixazomib can be administered without regard to the timing of dialysis.
Across all treatment cycles, five of 18 response‐evaluable patients (28%) with RRMM achieved a PR; all five of these patients had severe RI and 2 were refractory to prior PI therapy. However, the preliminary nature of these data should be noted due to the short treatment exposure, low number of treatment cycles and limited follow‐up period. In addition, the results are based on a heavily pre‐treated patient population.
The safety profile of once‐weekly ixazomib observed here was consistent with previous studies in patients with MM (Assouline et al, 2014; Kumar et al, 2014a,b; Richardson et al, 2014; Gupta et al, 2015a) and suggests that oral ixazomib administered once weekly leads to TEAEs that are generally manageable and reversible with dose modifications and standard supportive care. Although the incidence of the most common TEAEs (except for anaemia) was generally similar between renal function groups in the present study, the incidences of grade ≥3 TEAEs, SAEs and discontinuations due to AEs were higher for patients with severe RI or ESRD as compared with patients with normal renal function. In total, 94% of patients escalated from 3 mg to 4 mg at the start of Part B. All patients in the normal renal function and severe RI groups and 67% of patients in the ESRD group received 4 mg ixazomib at the start of Part B and there was a similar proportion of patients with dose reductions in the severe RI/ESRD combined group versus patients with normal renal function. However, the relative dose intensity was much lower in patients with severe RI or ESRD due to the higher rate of permanent discontinuations which, based on the PK data, may be at least partly due to higher systemic exposures in these patients. In addition, patients in the severe RI and ESRD groups had received more lines of prior therapy, and more patients were refractory to prior proteasome inhibitor and IMiD‐based therapies than in the normal renal function group.
Although the use of dexamethasone was allowed at the discretion of the investigator in Part B, all PK assessments were based on Part A of the study. Furthermore, a pooled PK analysis from ixazomib monotherapy and combination studies across various indications identified no PK interaction between ixazomib and dexamethasone (Gupta et al, 2013). Nevertheless, it should be considered that the optional use of dexamethasone in some patients with RRMM in Part B of the present study might have influenced the safety and efficacy results.
In conclusion, based on the PK and safety findings, a reduced 3 mg dose of ixazomib (on days 1, 8 and 15 of 28‐d cycles) is recommended for MM patients with severe RI or ESRD requiring haemodialysis, relative to the standard 4 mg dose that is recommended for patients with normal renal function or mild or moderate RI (Gupta et al, 2015b). In patients with ESRD requiring haemodialysis, ixazomib can be administered irrespective of the timing of dialysis.
Author contributions
NG, MJH and AMH designed the study, performed the research, analysed and interpreted the data, and reviewed and revised the manuscript. RDH performed the research, contributed to patient recruitment, interpreted the data, and reviewed and revised the manuscript. AB, BL, VK, and JB performed the research, interpreted the data, and reviewed and revised the manuscript. HY, MQ and XZ performed the research, analysed and interpreted the data, and reviewed and revised the manuscript. KV and AC designed the study, performed the research, interpreted the data, and reviewed and revised the manuscript. All authors approved the final version.
Disclosure and competing interests statement
NG, MJH, HY, MQ, XZ, and KV are employees of Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. RDH has received research funding from and has been an advisor for Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company. VK has received honoraria from Celgene, Janssen Ortho, Lundbeck, and Amgen. JB has received research funding from MEI, Celgene, Bristol‐Myers Squibb, Onyx, Janssen, Novartis, AbbVie, Curis, Acetylon, Array, and Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. AMH is a former employee of Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. AC has been an advisor for Celgene and has received research funding from and been a consultant for Array BioPharma, Celgene, Novartis, Onyx, and Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. AB and BL have no financial relationships to disclose.
Acknowledgements
The authors would like to acknowledge Susan Chen, the laboratory analyst who conducted the plasma protein binding assay, and Mike Bargfrede, for final non‐compartmental analysis to estimate PK parameters. Writing support during the development of this manuscript was provided by Fiona Scott, a medical writer with FireKite, an Ashfield company, part of UDG Healthcare plc, which was funded by Millennium Pharmaceuticals, Inc., and complied with Good Publication Practice 3 ethical guidelines (Battisti et al, 2015, Ann Int Med, 163, 461‐464).
Financial support: This work was funded by Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
This work has been presented at the following meetings: Poster presented at the 57th American Society of Hematology Annual Meeting, Orlando, Florida, USA, December 5–8, 2015.
References
- Assouline, S.E. , Chang, J. , Cheson, B.D. , Rifkin, R. , Hamburg, S. , Reyes, R. , Hui, A.M. , Yu, J. , Gupta, N. , Di, B.A. , Shou, Y. & Martin, P. (2014) Phase 1 dose‐escalation study of IV ixazomib, an investigational proteasome inhibitor, in patients with relapsed/refractory lymphoma. Blood Cancer J., 4, e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badros, A.Z. , Vij, R. , Martin, T. , Zonder, J.A. , Kunkel, L. , Wang, Z. , Lee, S. , Wong, A.F. & Niesvizky, R. (2013) Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia, 27, 1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti, W.P. , Wager, E. , Baltzer, L. , Bridges, D. , Cairns, A. , Carswell, C.I. , Citrome, L. , Gurr, J.A. , Mooney, L.A. , Moore, B.J. , Peña, T. , Sanes‐Miller, C.H. , Veitch, K. , Woolley, K.L. & Yarker, Y.E. ; International Society for Medical Publication Professionals . (2015) Good Publication Practice for Communicating Company‐Sponsored Medical Research: GPP3. Annals of Internal Medicine, 163, 461–464. [DOI] [PubMed] [Google Scholar]
- Chanan‐Khan, A.A. , San Miguel, J.F. , Jagannath, S. , Ludwig, H. & Dimopoulos, M.A. (2012) Novel therapeutic agents for the management of patients with multiple myeloma and renal impairment. Clinical Cancer Research, 18, 2145–2163. [DOI] [PubMed] [Google Scholar]
- Chen, N. , Lau, H. , Kong, L. , Kumar, G. , Zeldis, J.B. , Knight, R. & Laskin, O.L. (2007) Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. Journal of Clinical Pharmacology, 47, 1466–1475. [DOI] [PubMed] [Google Scholar]
- Clark, A.D. , Shetty, A. & Soutar, R. (1999) Renal failure and multiple myeloma: pathogenesis and treatment of renal failure and management of underlying myeloma. Blood Reviews, 13, 79–90. [DOI] [PubMed] [Google Scholar]
- Cockcroft, D.W. & Gault, M.H. (1976) Prediction of creatinine clearance from serum creatinine. Nephron, 16, 31–41. [DOI] [PubMed] [Google Scholar]
- Dahlke, M. , Halabi, A. , Canadi, J. , Tsubouchi, C. , Machineni, S. & Pang, Y. (2016) Pharmacokinetics of serelaxin in patients with severe renal impairment or end‐stage renal disease requiring hemodialysis: a single‐dose, open‐label, parallel‐group study. Journal of Clinical Pharmacology, 56, 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos, M.A. , Richardson, P.G. , Schlag, R. , Khuageva, N.K. , Shpilberg, O. , Kastritis, E. , Kropff, M. , Petrucci, M.T. , Delforge, M. , Alexeeva, J. , Schots, R. , Masszi, T. , Mateos, M.V. , Deraedt, W. , Liu, K. , Cakana, A. , van de Velde, H. & San Miguel, J.F. (2009a) VMP (Bortezomib, Melphalan, and Prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. Journal of Clinical Oncology, 27, 6086–6093. [DOI] [PubMed] [Google Scholar]
- Dimopoulos, M.A. , Roussou, M. , Gavriatopoulou, M. , Zagouri, F. , Migkou, M. , Matsouka, C. , Barbarousi, D. , Christoulas, D. , Primenou, E. , Grapsa, I. , Terpos, E. & Kastritis, E. (2009b) Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib‐based regimens: identification of predictive factors. Clin. Lymphoma Myeloma., 9, 302–306. [DOI] [PubMed] [Google Scholar]
- Dimopoulos, M.A. , Terpos, E. , Chanan‐Khan, A. , Leung, N. , Ludwig, H. , Jagannath, S. , Niesvizky, R. , Giralt, S. , Fermand, J.P. , Blade, J. , Comenzo, R.L. , Sezer, O. , Palumbo, A. , Harousseau, J.L. , Richardson, P.G. , Barlogie, B. , Anderson, K.C. , Sonneveld, P. , Tosi, P. , Cavo, M. , Rajkumar, S.V. , Durie, B.G. & San Miguel, J.F. (2010) Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. Journal of Clinical Oncology, 28, 4976–4984. [DOI] [PubMed] [Google Scholar]
- Durie, B.G. , Harousseau, J.L. , Miguel, J.S. , Blade, J. , Barlogie, B. , Anderson, K. , Gertz, M. , Dimopoulos, M. , Westin, J. , Sonneveld, P. , Ludwig, H. , Gahrton, G. , Beksac, M. , Crowley, J. , Belch, A. , Boccadaro, M. , Cavo, M. , Turesson, I. , Joshua, D. , Vesole, D. , Kyle, R. , Alexanian, R. , Tricot, G. , Attal, M. , Merlini, G. , Powles, R. , Richardson, P. , Shimizu, K. , Tosi, P. , Morgan, G. & Rajkumar, S.V. (2006) International uniform response criteria for multiple myeloma. Leukemia, 20, 1467–1473. [DOI] [PubMed] [Google Scholar]
- EMEA . (2004) Note for Guidance on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients with Impaired Renal Function. European Medicines Agency Committee for Medicinal Products for Human Use European Medicines Agency. London, UK. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003123.pdf. Accessed July 17 2015.
- Gonsalves, W.I. , Leung, N. , Rajkumar, S.V. , Dispenzieri, A. , Lacy, M.Q. , Hayman, S.R. , Buadi, F.K. , Dingli, D. , Kapoor, P. , Go, R.S. , Lin, Y. , Russell, S.J. , Lust, J.A. , Zeldenrust, S. , Kyle, R.A. , Gertz, M.A. & Kumar, S.K. (2015) Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer Journal, 5, e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, N. , Noe, D. , Liu, G. , Berg, D. , Kalebic, T. , Shou, Y. , Hui, A.M. & Venkatakrishnan, K. (2013) Clinical pharmacokinetics (PK) of intravenous (IV) and oral MLN9708, an investigational proteasome inhibitor: pooled analysis from monotherapy and combination studies across various indications. Clinical Pharmacology and Therapeutics, 93, S32. Abstract P1‐51. [Google Scholar]
- Gupta, N. , Goh, Y.T. , Min, C.K. , Lee, J.H. , Kim, K. , Wong, R.S. , Chim, C.S. , Hanley, M.J. , Yang, H. , Venkatakrishnan, K. , Hui, A.M. , Esseltine, D.L. & Chng, W.J. (2015a) Pharmacokinetics and safety of ixazomib plus lenalidomide‐dexamethasone in Asian patients with relapsed/refractory myeloma: a phase 1 study. Journal of Hematology & Oncology, 8, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, N. , Zhao, Y. , Hui, A.M. , Esseltine, D.L. & Venkatakrishnan, K. (2015b) Switching from body surface area‐based to fixed dosing for the investigational proteasome inhibitor ixazomib: a population pharmacokinetic analysis. British Journal of Clinical Pharmacology, 79, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, N. , Hanley, M.J. , Venkatakrishnan, K. , Wang, B. , Sharma, S. , Bessudo, A. , Hui, A.M. & Nemunaitis, J. (2016) The effect of a high‐fat meal on the pharmacokinetics of ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors or lymphoma. Journal of Clinical Pharmacology, doi:10.1002/jcph.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Myeloma Working Group (2003) Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. British Journal of Haematology, 121, 749–757. [PubMed] [Google Scholar]
- Khadzhynov, D. , Slowinski, T. , Lieker, I. , Neumayer, H.H. , Albrecht, D. , Streefkerk, H.J. , Rebello, S. & Peters, H. (2012) Pharmacokinetics of aliskiren in patients with end‐stage renal disease undergoing haemodialysis. Clinical Pharmacokinetics, 51, 661–669. [DOI] [PubMed] [Google Scholar]
- Khan, R. , Apewokin, S. , Grazziutti, M. , Yaccoby, S. , Epstein, J. , vanRhee, F. , Rosenthal, A. , Waheed, S. , Usmani, S. , Atrash, S. , Kumar, S. , Hoering, A. , Crowley, J. , Shaughnessy, J.D., Jr & Barlogie, B. (2015) Renal insufficiency retains adverse prognostic implications despite renal function improvement following Total Therapy for newly diagnosed multiple myeloma. Leukemia, 29, 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen, L.M. , Hjorth, M. & Hippe, E. (2000) Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. European Journal of Haematology, 65, 175–181. [DOI] [PubMed] [Google Scholar]
- Kumar, S. (2010) Multiple myeloma ‐ current issues and controversies. Cancer Treatment Reviews, 36, S3–S11. [DOI] [PubMed] [Google Scholar]
- Kumar, S.K. , Bensinger, W.I. , Zimmerman, T.M. , Reeder, C.B. , Berenson, J.R. , Berg, D. , Hui, A.M. , Gupta, N. , Di, B.A. , Yu, J. , Shou, Y. & Niesvizky, R. (2014a) Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood, 124, 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S.K. , Berdeja, J.G. , Niesvizky, R. , Lonial, S. , Laubach, J.P. , Hamadani, M. , Stewart, A.K. , Hari, P. , Roy, V. , Vescio, R. , Kaufman, J.L. , Berg, D. , Liao, E. , Di, B.A. , Estevam, J. , Gupta, N. , Hui, A.M. , Rajkumar, V. & Richardson, P.G. (2014b) Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open‐label phase 1/2 study. Lancet Oncology, 15, 1503–1512. [DOI] [PubMed] [Google Scholar]
- Laing, A.A. , Geddes, C. & Soutar, R. (2015) Renal impairment at presentation in multiple myeloma continues to be associated with poor survival. British Journal of Haematology, 169, 901–902. [DOI] [PubMed] [Google Scholar]
- Ludwig, H. , Adam, Z. , Hajek, R. , Greil, R. , Tothova, E. , Keil, F. , Autzinger, E.M. , Thaler, J. , Gisslinger, H. , Lang, A. , Egyed, M. , Womastek, I. & Zojer, N. (2010) Light chain‐induced acute renal failure can be reversed by bortezomib‐doxorubicin‐dexamethasone in multiple myeloma: results of a phase II study. Journal of Clinical Oncology, 28, 4635–4641. [DOI] [PubMed] [Google Scholar]
- Merlini, G. , Sanchorawala, V. , Jeffrey, Z.A. , Kukreti, V. , Schoenland, S.O. , Jaccard, A. , Dispenzieri, A. , Cohen, A.D. , Berg, D. , Yuan, Z. , Hui, A.M. , Giovanni, P. & Comenzo, R.L. (2014) Long‐Term outcome of a phase 1 study of the investigational oral proteasome inhibitor (PI) ixazomib at the recommended phase 3 dose (RP3D) in patients (Pts) with relapsed or refractory systemic light‐chain (AL) amyloidosis (RRAL). Blood, 124, 3450.25293779 [Google Scholar]
- Mimura, N. , Hideshima, T. & Anderson, K.C. (2015) Novel therapeutic strategies for multiple myeloma. Experimental Hematology, 43, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito, F. , Gentile, M. , Mazzone, C. , Rossi, D. , Di, R.F. , Bringhen, S. , Ria, R. , Offidani, M. , Patriarca, F. , Nozzoli, C. , Petrucci, M.T. , Benevolo, G. , Vincelli, I. , Guglielmelli, T. , Grasso, M. , Marasca, R. , Baldini, L. , Montefusco, V. , Musto, P. , Cascavilla, N. , Majolino, I. , Musolino, C. , Cavo, M. , Boccadoro, M. & Palumbo, A. (2011) Safety and efficacy of bortezomib‐melphalan‐prednisone‐thalidomide followed by bortezomib‐thalidomide maintenance (VMPT‐VT) versus bortezomib‐melphalan‐prednisone (VMP) in untreated multiple myeloma patients with renal impairment. Blood, 118, 5759–5766. [DOI] [PubMed] [Google Scholar]
- Moreau, P. (2012) The future of therapy for relapsed/refractory multiple myeloma: emerging agents and novel treatment strategies. Seminars in Hematology, 49, S33–S46. [DOI] [PubMed] [Google Scholar]
- Moreau, P. , Masszi, T. , Grzasko, N. , Bahlis, N.J. , Hansson, M. , Pour, L. , Sandhu, I. , Ganly, P. , Baker, B.W. , Jackson, S. , Stoppa, A.M. , Simpson, D.R. , Gimsing, P. , Palumbo, A. , Garderet, L. , Cavo, M. , Kumar, S.K. , Touzeau, C. , Buadi, F. , Laubach, J.P. , Lin, J. , Berg, D. , DiBacco, A. , Hui, A.M. & Richardson, P.G. (2015a) Ixazomib, an investigational oral proteasome inhibitor (PI), in Combination with Lenalidomide and Dexamethasone (IRd), Significantly Extends Progression‐Free Survival (PFS) for Patients (Pts) with Relapsed and/or Refractory Multiple Myeloma (RRMM): the PGǪ. Blood, 126, 727. [Google Scholar]
- Moreau, P. , Pylypenko, H. , Grosicki, S. , Karamanesht, I. , Leleu, X. , Rekhtman, G. , Masliak, Z. , Robak, P. , Esseltine, D.L. , Feng, H. , Deraedt, W. , van de Velde, H. & Arnulf, B. (2015b) Subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma: subanalysis of patients with renal impairment in the phase III MMY‐3021 study. Haematologica, 100, e207–e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolin, T.D. , Naud, J. , Leblond, F.A. & Pichette, V. (2008) Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clinical Pharmacology and Therapeutics, 83, 898–903. [DOI] [PubMed] [Google Scholar]
- Penfield, J.G. (2006) Multiple myeloma in end‐stage renal disease. Seminars in Dialysis, 19, 329–334. [DOI] [PubMed] [Google Scholar]
- Richardson, P.G. , Baz, R. , Wang, M. , Jakubowiak, A.J. , Laubach, J.P. , Harvey, R.D. , Talpaz, M. , Berg, D. , Liu, G. , Yu, J. , Gupta, N. , Di, B.A. , Hui, A.M. & Lonial, S. (2014) Phase 1 study of twice‐weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood, 124, 1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Miguel, J.F. , Schlag, R. , Khuageva, N.K. , Dimopoulos, M.A. , Shpilberg, O. , Kropff, M. , Spicka, I. , Petrucci, M.T. , Palumbo, A. , Samoilova, O.S. , Dmoszynska, A. , Abdulkadyrov, K.M. , Schots, R. , Jiang, B. , Mateos, M.V. , Anderson, K.C. , Esseltine, D.L. , Liu, K. , Cakana, A. , van de Velde, H. & Richardson, P.G. (2008) Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. New England Journal of Medicine, 359, 906–917. [DOI] [PubMed] [Google Scholar]
- Scheid, C. , Sonneveld, P. , Schmidt‐Wolf, I.G. , van der Holt, B. , el Jarari, L. , Bertsch, U. , Salwender, H. , Zweegman, S. , Blau, I.W. , Vellenga, E. , Weisel, K. , Pfreundschuh, M. , Jie, K.S. , Neben, K. , van de Velde, H , Duehrsen, U. , Schaafsma, M.R. , Lindemann, W. , Kersten, M.J. , Peter, N. , Hanel, M. , Croockewit, S. , Martin, H. , Wittebol, S. , Bos, G.M. , van Marwijk‐Kooy, M. , Wijermans, P. , Goldschmidt, H. & Lokhorst, H.M. (2014) Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON‐65/GMMG‐HD4 trial. Haematologica, 99, 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D.C. , Kalebic, T. , Infante, J.R. , Siu, L.L. , Sullivan, D. , Vlahovic, G. , Kauh, J.S. , Gao, F. , Berger, A.J. , Tirrell, S. , Gupta, N. , Di, B.A. , Berg, D. , Liu, G. , Lin, J. , Hui, A.M. & Thompson, J.A. (2015) Phase 1 study of ixazomib, an investigational proteasome inhibitor, in advanced non‐hematologic malignancies. Investigational New Drugs, 33, 652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, A.K. , Rajkumar, S.V. , Dimopoulos, M.A. , Masszi, T. , Spicka, I. , Oriol, A. , Hajek, R. , Rosinol, L. , Siegel, D.S. , Mihaylov, G.G. , Goranova‐Marinova, V. , Rajnics, P. , Suvorov, A. , Niesvizky, R. , Jakubowiak, A.J. , San‐Miguel, J.F. , Ludwig, H. , Wang, M. , Maisnar, V. , Minarik, J. , Bensinger, W.I. , Mateos, M.V. , Ben‐Yehuda, D. , Kukreti, V. , Zojwalla, N. , Tonda, M.E. , Yang, X. , Xing, B. , Moreau, P. & Palumbo, A. (2015) Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. The New England Journal of Medicine, 372, 142–152. [Google Scholar]
- Sun, H. , Frassetto, L. & Benet, L.Z. (2006) Effects of renal failure on drug transport and metabolism. Pharmacology & Therapeutics, 109, 1–11. [DOI] [PubMed] [Google Scholar]
- Tosi, P. , Imola, M. , Mianulli, A.M. , Tomassetti, S. , Molinari, A. , Mangianti, S. , Ratta, M. , Merli, A. & Polli, V. (2015) Multiple myeloma and renal failure. European Medical Journal, 3, 65–69. [Google Scholar]
- US FDA (2010). Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function ‐ Study Design, Data Analysis, and Impact on Dosing and Labeling. US Food and Drug Admnistration, Rockville: http://www.fda.gov/downloads/Drugs/.../Guidances/UCM204959.pdf. Accessed July 22 2015. [Google Scholar]
- Zhang, Y. , Zhang, L. , Abraham, S. , Apparaju, S. , Wu, T.C. , Strong, J.M. , Xiao, S. , Atkinson, A.J. Jr , Thummel, K.E. , Leeder, J.S. , Lee, C. , Burckart, G.J. , Lesko, L.J. & Huang, S.M. (2009) Assessment of the impact of renal impairment on systemic exposure of new molecular entities: evaluation of recent new drug applications. Clinical Pharmacology and Therapeutics, 85, 305–311. [DOI] [PubMed] [Google Scholar]


