Figure 1.
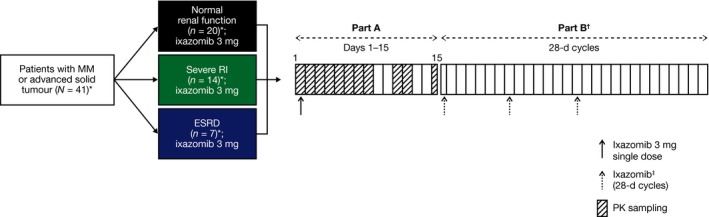
Study design overview. *Safety population. The PK‐evaluable population included all patients who received the protocol‐specified dose of ixazomib in Part A, did not receive any excluded concomitant medications through the completion of PK sampling, and had sufficient concentration–time data to permit reliable estimation of PK parameters by non‐compartmental analysis methods (normal renal function, n = 18, severe RI, n = 14, ESRD, n = 6); †Started on Day 15 after PK sample collection in Part A; ‡Patients received ixazomib at a dose of 4, 3, or 2·3 mg on Days 1, 8, and 15 of each 28‐d cycle in Part B, depending on the tolerability of treatment in Part A. MM, multiple myeloma; ESRD, end‐stage renal disease requiring haemodialysis; PK, pharmacokinetics; RI, renal impairment.
