Figure 6.
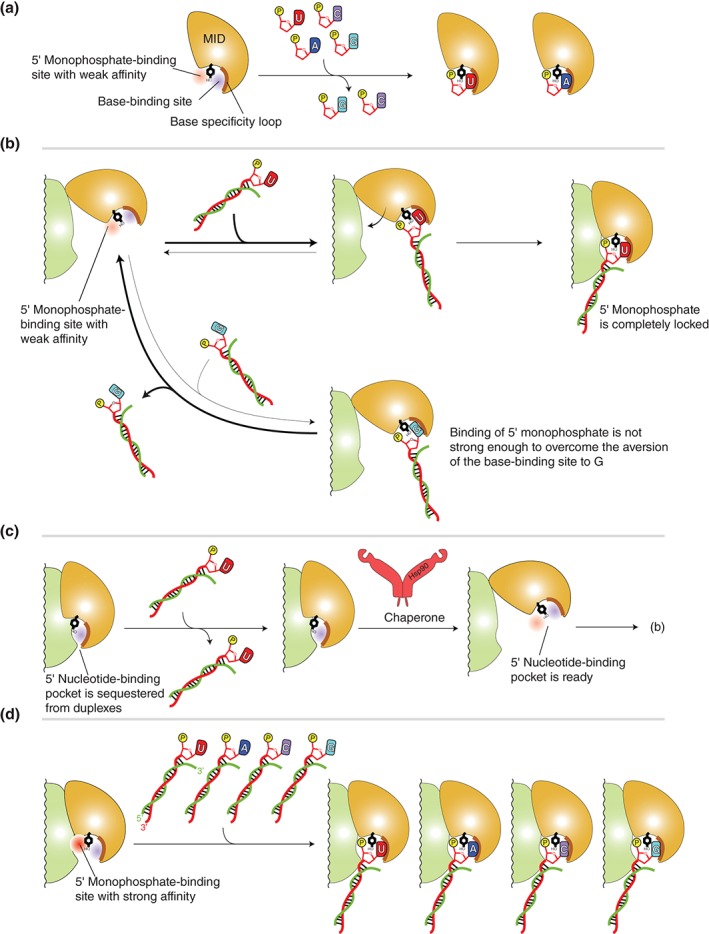
Models of stepwise duplex loading. (a) Interaction between the MID domain alone with nucleoside monophosphates, UMP (red), AMP (blue), CMP (magenta), and GMP (cyan). The MID domain preferentially binds U and A over C and G. The 5′ nucleotide‐binding pocket is divided into the 5′ monophosphate (red)‐ and the base (blue)‐binding sites by a conserved tyrosine residue (black) that is stacked with the 5′ base and its adjacent monophosphate (yellow). The base specificity loop is shown as a thick line (brown). (b) Model of sorting guide strand by the affinity of the MID domain to the 5′ base. Top: The MID domain alone binds to the uracil (or adenine) at the 5′ position of duplexes. The interaction is stable enough to endure until it forms the composite pocket with the PIWI domain. Bottom: The guanine (or cytosine) at the 5′ position of duplexes can bind to the 5′ base‐binding pocket. Owing to the aversion of the nucleotide specificity loop to cytosine and guanine, most of the complexes are dissociated before they form the composite pocket with PIWI domain. Therefore, the duplexes including guanine (or cytosine) at their 5′ position are easily released from the AGO protein. (c) Autoinhibition model. The MID and PIWI domains of unloaded AGO protein interact with each other such that the 5′ nucleotide‐binding pocket is not accessible. Chaperone machinery may pry open the autoinhibited conformation, and the resultant open pocket proceeds to (b). (d) Model of preorganized composite pocket. The MID and PIWI domains of unloaded AGO protein already complete the 5′ monophosphate‐binding site whose affinity is higher than that of the MID domain alone (a). The affinity to the 5′ monophosphate would overwhelm the aversion of the base‐binding site to guanine and cytosine. As a result, the preorganized composite pocket can bind any duplexes, regardless of the types of the 5′ base.
