Figure 8.
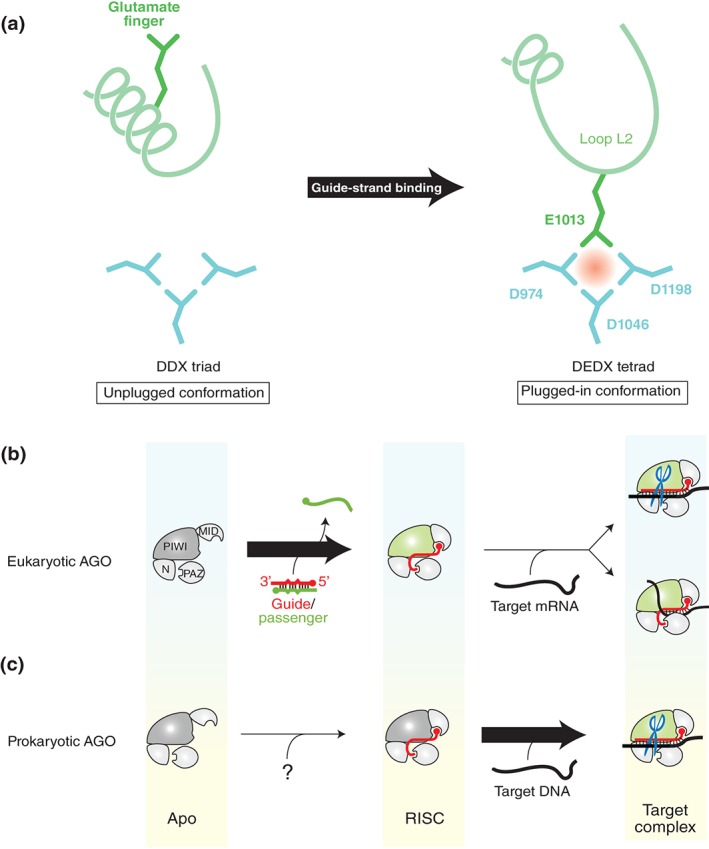
Difference between eukaryotic and prokaryotic AGO proteins. (a) Conformational change activates the AGO protein. In the catalytically inactive state (i.e., unplugged conformation), the region including the glutamate finger (green) folds into an α‐helix (left panel). In the active state (i.e., plugged‐in conformation), the α‐helix is partially unfolded and the glutamate finger is inserted into the DDX triad (aqua) to complete the catalytic DEDX tetrad (right panel). The residue numbers of KpAGO are indicated. (b and c) Requirements for the transition to the plugged‐in conformation. Eukaryotic AGO proteins transition to the plugged‐in state during the RISC assembly (b), whereas the prokaryotic counterparts need to incorporate a target strand to be plugged in (c). The source of guide strand for the prokaryotic AGO proteins remains unknown. The PIWI domain colored in green indicates the plugged‐in conformation.
