Figure 4.
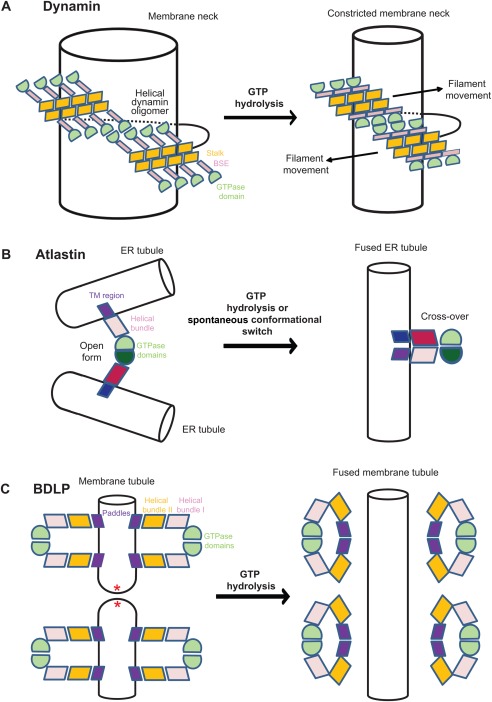
Schematic models for the mechano‐chemical action of dynamin superfamily proteins on membranes. The same domain colouring scheme as in Figures 1–3 is used (A) Constriction model of dynamin.100 After PH‐domain dependent recruitment to the membrane, a right‐handed dynamin filament oligomerizes via the stalk around a tubular membrane template, like the neck of clathrin‐coated vesicles. Once the filament has embraced the tubular membrane template, GTPase domains of adjacent helical turns dimerize. The GTPase‐induced power stroke was suggested to pull adjacent filaments along each other (arrow) leading to constriction and eventually cleavage of the membrane tubule. For better clarity, the PH domains are not included in this graph. (B) Fusion model of atlastin. The GTPase domains of atlastin dimerize across ER tubules in the open conformation. Nucleotide hydrolysis‐induced conformational changes catalyze the transition toward the cross‐over conformation, thereby pulling the two membranes toward each other, leading to membrane fusion. Note that the indicated transmembrane domains (blue) were not present in the crystallized construct. (C) Fusion model of BDLP. In the GTP‐bound form, BDLP oligomerizes in its open conformation on a membrane surface, thereby inducing and stabilizing membrane tubules of high curvature, probably by insertion of the paddle region. GTP hydrolysis converts BDLP back to the closed conformation which is released from the membrane, leaving behind highly strained membrane tubule, that are prone to undergo membrane fusion (according to Ref. 39).
