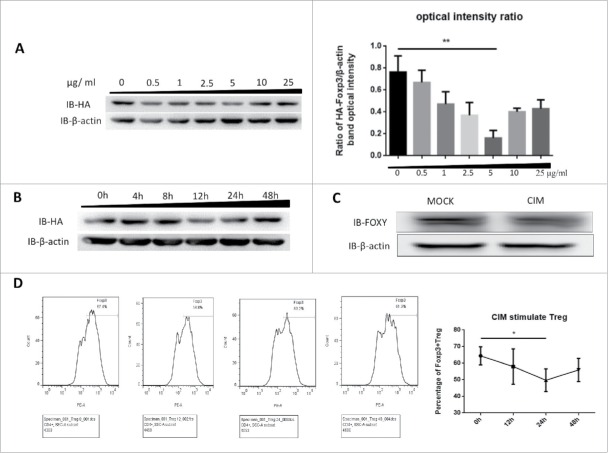
Figure 1.
Foxp3 is destabilized by stimulation with cimetidine. A. Decrease in Foxp3 in Jurkat cells stimulated with different doses of CIM. HA-Foxp3 Jurkat T cells were adjusted to 2×106/ml, and stimulated with 0.5, 1, 2.5, 5, 10, 25 μg/ml cimetidine for 12 h. Four hours before the end of incubation, PMA (50 ng/ml) and ionomycin (1 μg/ml) were added to activate the stimulated cells. The level of Foxp3 protein in cell lysates was detected by immunoblotting (Left). The expression relative to β-actin was analyzed as intensity ratio by Image-Pro Plus, HA/β-actin (Right). B. CIM-induced Foxp3 loss in Jurkat cells treated for different length of time. HA-Foxp3 Jurkat T cells were adjusted to 2×106/ml, and stimulated with cimetidine (5 μg/ml) for 4, 8, 12, 24, or 48 h. PMA (50 ng/ml) and ionomycin (1 μg/ml) were added 4 h before cell harvest. Foxp3 protein in cell lysates was normalized and detected by immunoblotting. C. Decrease in Foxp3 in CIM-stimulated primary human Treg cells. Human primary Treg cells were purified from peripheral blood mononuclear cells of healthy donors at the Shanghai Blood Center and separated by fluorescence cell sorting (FACS) to obtain CD4+CD25hiCD127lo Treg cells. The Treg were then stimulated with cimetidine (5 μg/ml) for 12 h. Foxp3 protein in cell lysates was immunoblotted with FOXY-antibody. D. CIM-induced decrease in the percentage of primary human Treg cells that stained with Foxp3. Human Treg cells stimulated with CIM were stained with CD4, CD25 and CD127 and analyzed by FACS. The sequential changes in percentage of Foxp3+ cells during stimulation are summarized. Data are expressed as means SD from 3 independent experiment. Statistical significance was calculated using t-test (**P < 0.01 and *P < 0.05).