Figure 6.
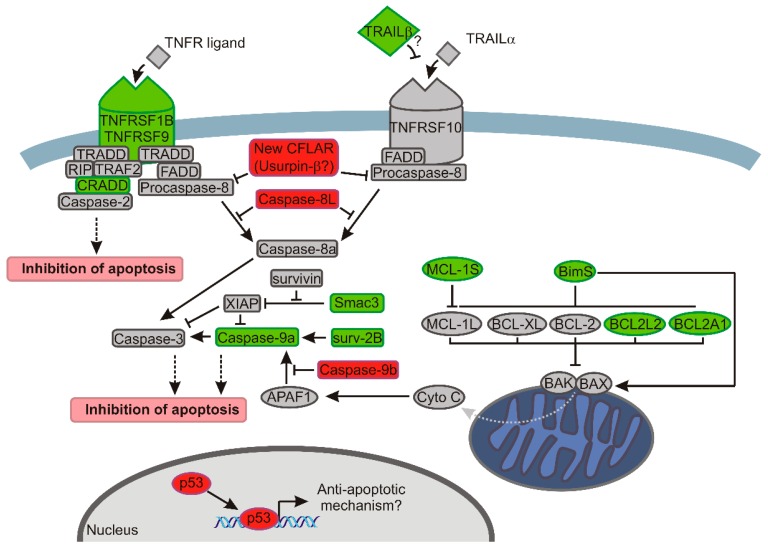
The model showing how decreased expression of SRSF2 affects apoptotic pathways in renal cancer cells. The changes introduced by the depletion of SRSF2 are shown with the following colours: green, decreased expression; and red, increased expression. The arrows indicate activation, bar-headed lines indicate inhibition. Grey dotted arrow indicates release of cytochrome c. Black dotted arrows indicate final effect on apoptosis. In the extrinsic apoptotic pathway (left side of the drawing) depletion of SRSF2 results in downregulated expression of genes coding for death receptors (TNFRSF1B and TNFRSF9) and CRADD that recruits caspase-2 to complex with death receptors. Additionally, the expression of TRAIL-β, a non-active splice variant of a ligand of the TNFRSF10 receptor [48] is decreased. Overexpressed caspase-8L interferes with the binding of caspase-8a to FADD (Fas-associated death domain-containing protein) and counteracts its activation [49]. Likewise, caspase-8 activation is probably hindered by overexpression of the new splice variant of CFLAR, coding for Usurpin β [18]. In the intrinsic apoptotic pathway (right side of the drawing) BAX and BAK oligomerize, trigger permeabilization of the outer mitochondrial membrane and release of cytochrome c. The proapototic activity of BAX and BAK is counteracted by antiapoptotic proteins of BCL-2 family (MCL-1L, BCL-XL, BCL-2, BCL2L2, and BCL2A1) [14]. In cells with lowered expression of SRSF2, the concurrent decrease of antiapoptotic BCL2A1 and BCL2L2 might be overcome by diminished expression of proapoptotic BimS (the most powerful pro-apoptotic isoform of BIM variants) that inhibits the activity of antiapoptotic BCL2 proteins and directly activates BAX [22,23,24]. A decrease of BimS may, thus, lead to, on one hand, diminished activation of BAX, and on the other hand, to inefficient inhibition of BCL-2 proteins, allowing them to inhibit BAX and BAK. A similar effect is exerted by decreased expression of proapoptotic MCL-1S that acts as an inhibitor of antiapoptotic MCL-1L [21]. The antiapoptotic effect of SRSF2 depletion is possibly executed by diminished activity of caspase-9, resulting from: (1) decreased expression of caspase-9a; (2) increased expression of caspase-9b that inhibits Apaf-1-mediated activation of caspase-9a [50,51]; (3) decreased expression of Smac3 which inactivates XIAP that acts as caspases’ inhibitor; and (4) decreased expression of proapoptotic Surv-2B that acts as an activator of caspase-9 [20]. Notably, the expression of antiapoptotic survivin is not changed by SRSF2 silencing. Finally, decreased expression of SRSF2 results in upregulation of TP53, which inhibits apoptosis in renal cancer cells [38,39,40,41,52].