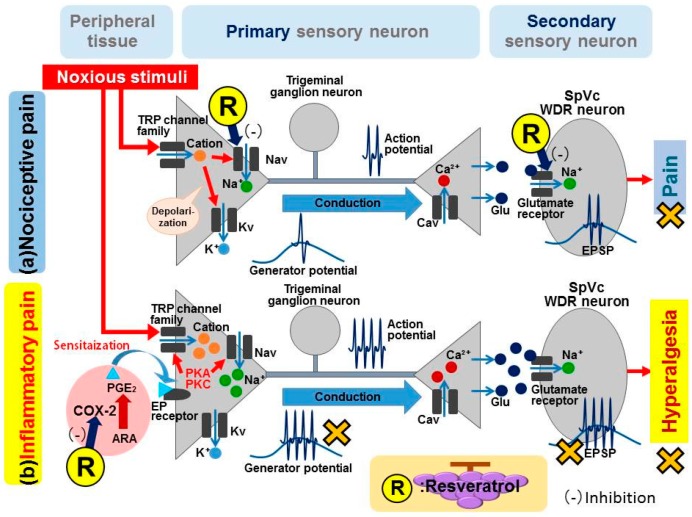
Figure 1.
Schematic drawing of the possible mechanism underlying the effects of resveratrol in relieving (a) nociceptive and(b) inflammatory pain. (a) nociceptive pain. When noxious mechanical stimulation is applied to the skin, mechanosensitive ion channels (e.g., transient receptor potential ankyrin 1 (TRPA1) channels) open, activating the generator potential (depolarization). This depolarization further opens voltage-dependent sodium and potassium channels, generating action potentials. Action potentials are discharged through primary afferent fibers (Aδ- and C-fibers) to the central terminal in nociceptive neurons in the trigeminal spinal nucleus caudalis (SpVc). When action potentials are conducted to the central terminal of the SpVc, presynaptic voltage-dependent calcium channels open, leading to the release of neurotransmitters (e.g., glutamate) into the synaptic cleft, which then bind to post-synaptic (glutamate) receptors, activating excitatory post-synaptic potentials (EPSP). If the amplitude of EPSPs is over the action potential threshold, a barrage of action potentials is conducted to higher centers in the pain pathway and pain is perceived. It is possible that resveratrol suppresses both the excitability of peripheral terminals of the trigeminal nerve (by modulating both the mechanical transduction and generation of action potentials) and glutaminergic excitatory synaptic transmission of the SpVc (by inhibiting post-synaptic glutamate receptors and presynaptic Ca2+ channels). R, resveratrol; Nav, voltage-gated sodium channel; Kv, voltage-gated potassium channel; Cav, voltage-gated calcium channel; Glu, glutamate; WDR, wide dynamic range neurons; (b) Inflammatory pain. Following peripheral inflammation and/or nerve injury, inflammatory mediators, such as prostaglandin E2 (PGE2), bind to G-protein-coupled E-type prostanoid (EP) receptors and induce activation of protein kinases A and C (PKA and PKC, respectively) in nociceptive peripheral terminals, leading to phosphorylation of mechanosensitive, sodium and potassium ion channels and receptors. As a result, the activation threshold for transducer channels such as TRPA1 is reduced and the membrane excitability of the peripheral terminals increases, resulting in a high frequency of action potentials being conducted to presynaptic central terminals of the SpVc. This results in the release of a large amount of glutamate into the synaptic cleft, which binds to upregulated post-synaptic glutamate receptors, augmenting EPSPs, causing a barrage of action potentials to be conducted to higher centers of pain pathways and creating a state of heightened sensitivity termed peripheral sensitization. It is possible that chronic administration of resveratrol attenuates inflammation-induced mechanical inflammatory hyperalgesia, with this effect due primarily to suppression of the hyperexcitability of SpVc WDR neurons via inhibition of both peripheral and central cyclooxygenase (COX)-2 cascade signaling pathways. ARA, arachidonic acid. X: Suppression.