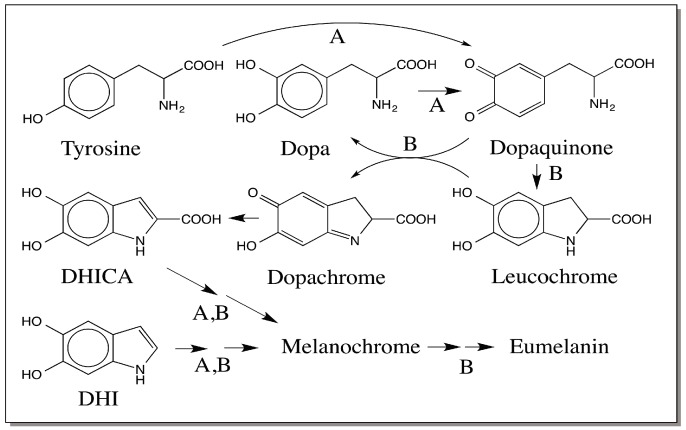
Figure 1.
Raper–Mason pathway for the biosynthesis of melanin. The bifunctional enzyme, tyrosinase (A) converts tyrosine and dopa to dopaquinone. Dopaquinone undergoes instantaneous intramolecular nonenzymatic cyclization forming leucochrome, which is rapidly oxidized by dopaquinone to dopachrome. The red colored dopachrome is converted to 5,6-dihydroxyindole (DHI) as the major product and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) as the minor product. Oxidative polymerization of dihydroxyindoles produces the melanin pigment. Tyrosinase is assumed to be the sole enzyme associated with this pathway and the rest of the reactions (B) are presumed to be of nonenzymatic origin.