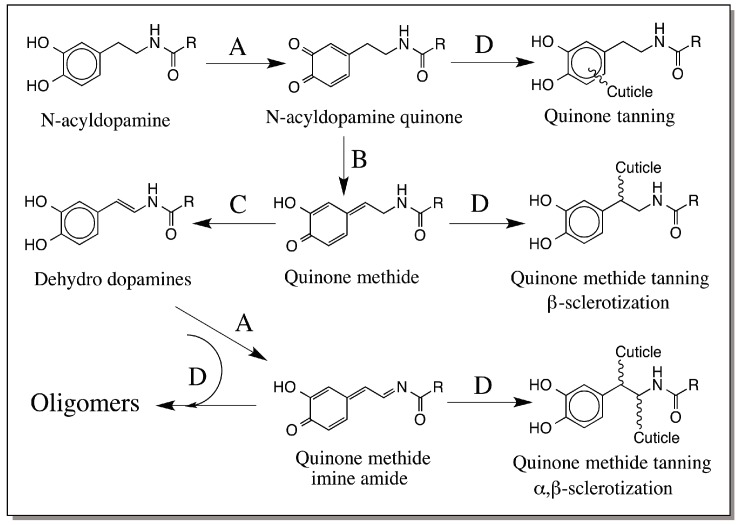
Figure 12.
Unified mechanism for sclerotization of insect cuticle. Sclerotizing precursors such as N-acetyldopamine (R = CH3) and N-β-alanyldopamine (R = CH2CH2NH2) are oxidized by cuticular phenoloxidases (A) to their corresponding quinones, which participate in quinone tanning reaction by forming Michael-1,4-addition reaction with cuticular nucleophiles. Quinone isomerase (B) converts part of the quinones to quinone methides and provide for quinone methide tanning through Michael-1,6-addition reactions. Some of the quinone methide also serves as substrate for quinone methide isomerase (C), which transforms them to 1,2-dehydro-N-acyldopamines. Oxidation of dehydro compounds by phenoloxidase generates the bifunctional quinone methide imine amides that form adducts with both the side chain carbon atoms. Some of the dehydro compound also undergoes oligomerization reaction (D = nonenzymatic reactions).