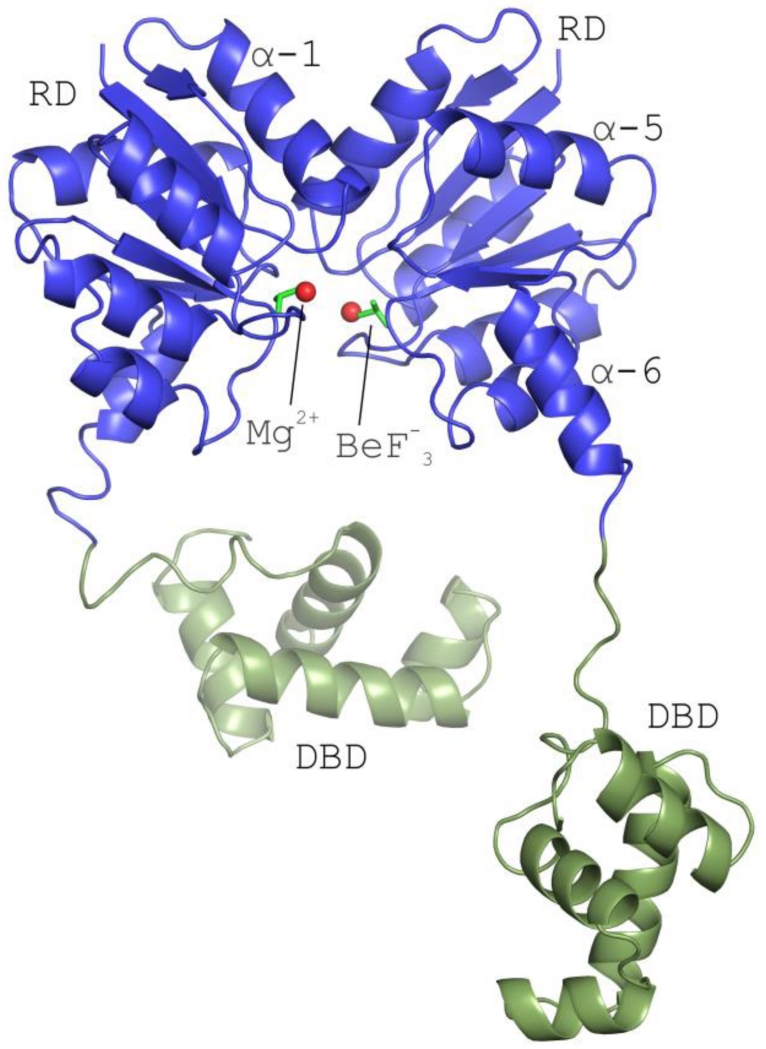
Figure 2. The structure of full-length Efm LiaR in complex with BeF3−/Mg2+ reveals an activated protein poised to bind DNA.
Structural overview of the full length Efm LiaR activated with the BeF3− (PDB ID: 5HEV). For clarity only one of the two pairs of LiaR dimers present in the asymmetric unit are displayed. Receiver domain (blue) showing a molecule of BeF3− bound proximal to the predicted phosphorylation site (Asp54). The LiaR dimerization surface is made up of the α-1 and α-5 helices. Mg2+ and BeF3− are shown as spheres (red) and sticks (dark green), respectively. Helix α-6 makes up part of the flexible linker joining the receiver and DNA-binding domains (light green). The flexible linker allows the DNA binding domains to have a substantial amount of conformational flexibility to engage and bind to DNA.