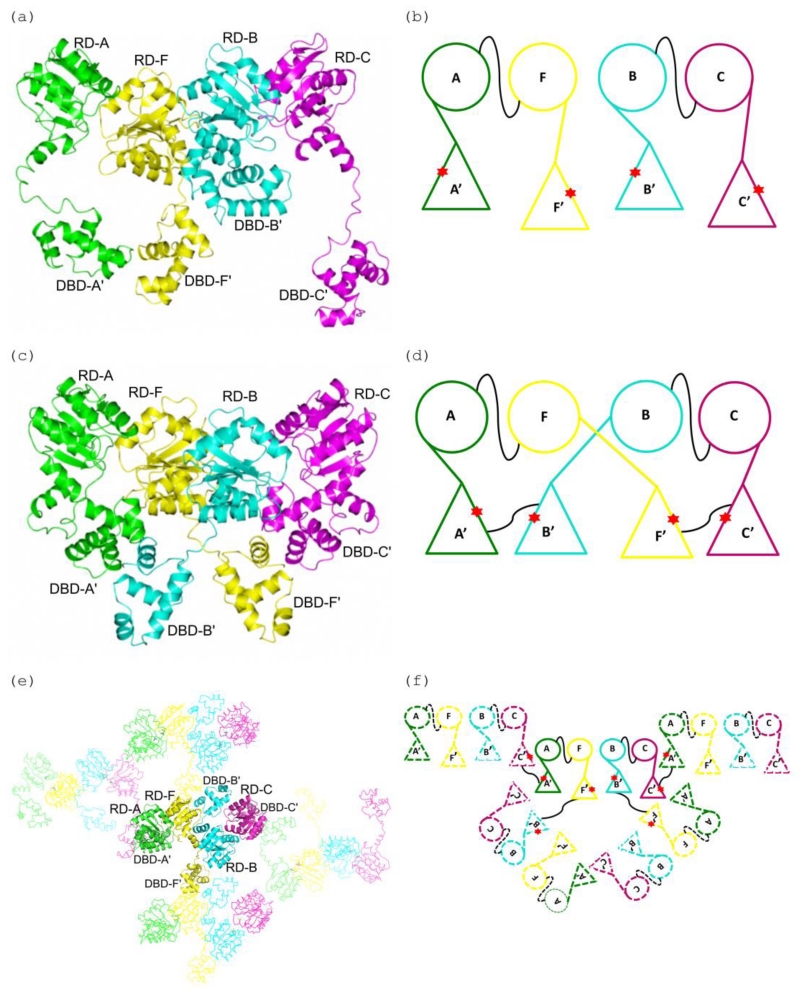
Figure 4. A comparison of the DNA domain swapping observed in the E. faecium LiaR and S. aureus VraR structures.
(a), (b) Cartoon depiction of the Efm LiaR structure (PDB ID: 5HEV) showing the receiver domain (RD) and DNA-binding (DBD) domains arrangements in the asymmetric unit. In the presence of BeF3− the crystal contained four protomers of LiaR in the asymmetric unit: A (green), F (yellow), B (cyan) and C (magenta). The star (red) identifies the dimerization interfaces of the DNA-binding domains. Two LiaR protomers (A-F and B-C) form a dimer through dimerization of their receiver domains. The thin black line connecting the monomers identifies the dimerization interfaces of the RDs of two protomers (A-F and B-C).
(c), (d) Cartoon representation of the domain swapped arrangement in the S. aureus VraR activated structure (PDB ID: 4IF4). Two VraR protomers form a dimer through dimerization of their receiver domains (A-F and B-C), while the DNA binding domains form homodimers with a DNA binding domain contributed from a different receiver domain (A’-B’ and F’-C’) resulting in a swapping of domains. The thin black line connecting the monomers identifies the dimerization interfaces.
(e), (f) Cartoon representation of the domain swapping arrangement for the Efm LiaR activated structure (PDB ID: 5HEV). Unlike VraR, the Efm LiaR DNA binding domains make dimer contacts to symmetry related DNA binding domains contributed by other receiver domain pairs. While this arrangement of swapped domains might be an artifact of crystallization it also demonstrates the flexibility of the DNA binding domains in the activated state. The thin black line connecting the monomers identifies the dimerization interfaces.