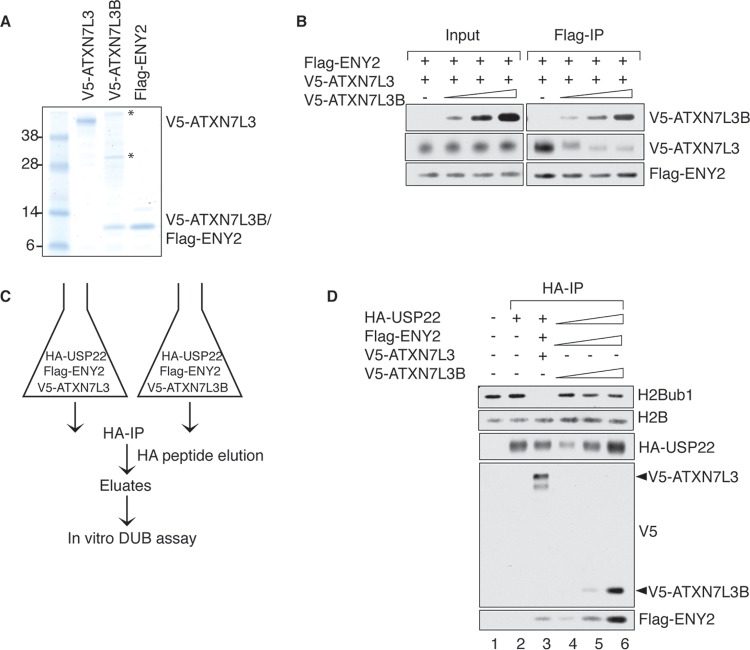
FIG 4.
ATXN7L3B competes with ATXN7L3 for ENY2 binding in vitro. (A) Recombinant proteins were purified from Sf21 cells and resolved by electrophoresis, followed by colloidal blue staining. Asterisks indicate background protein bands. (B) The indicated recombinant proteins were mixed in an in vitro competition reaction, and the formed complexes were precipitated with anti-Flag resin. The resin-bound proteins were eluted, resolved by SDS-PAGE, and detected by immunoblotting with the indicated antibodies. Five percent of the in vitro reactions was used as input. (C) Schematic of HA affinity purification using Sf21 cells coinfected with baculovirus containing expressing vector of HA-USP22, Flag-ENY2, and V5-ATXN7L3 or V5-ATXN7L3B. (D) The indicated immunoprecipitated complexes from panel C were used in in vitro deubiquitination reactions in which purified histones served as the substrates. Proteins in reactions were resolved by electrophoresis and detected by immunoblotting with the indicated antibodies.