ABSTRACT
Gram-positive rubber degraders such as Streptomyces sp. strain K30 cleave rubber [poly(cis-1,4-isoprene)] to low-molecular-mass oligoisoprenoid products with terminal keto and aldehyde groups by the secretion of a latex clearing protein (Lcp) designated rubber oxygenase. LcpK30 is a heme b cytochrome and has a domain of unknown function (DUF2236) that is characteristic of orthologous Lcps. Proteins with a DUF2236 domain are characterized by three highly conserved residues (R164, T168, and H198 in LcpK30). Exchange of R164 or T168 by alanine and characterization of the purified LcpK30 muteins revealed that both were stable and contained a heme group (red color) but were inactive. This finding identifies both residues as key residues for the cleavage reaction. The purified H198A mutein was also inactive and stable but was colorless due to the absence of heme. We constructed and characterized alanine muteins of four additional histidine residues moderately conserved in 495 LcpK30 homologous sequences (H203A, H232A, H259A, H266A). All muteins revealed wild-type properties, excluding any importance for activity and/or heme coordination. Since LcpK30 has only eight histidines and the three remaining residues (H103, H184, and H296) were not conserved (<11%), H198 presumably is the only essential histidine, indicating its putative function as a heme ligand. The second axial position of the heme is likely occupied by a not yet identified molecule. Mutational analysis of three strictly conserved arginine residues (R195, R202, R328) showed that R195A and R202A muteins were colorless and instable, suggesting that these residues are important for the protein stability.
IMPORTANCE Large amounts of rubber waste materials have been permanently released into the environment for more than a century, yet accumulation of rubber particles released, e.g., by abrasion of tires along highways has not been observed. This is indicative of the ubiquitous presence and activity of rubber-degrading microorganisms. Despite increasing research activities on rubber biodegradation during the last 2 decades, the knowledge of the enzymatic cleavage mechanism of rubber by latex clearing protein (Lcp) still is limited. In particular, the catalytic cleavage mechanism and the amino acids of Lcp proteins (Lcps) that are involved have not yet been identified for any Lcp. In this study, we investigated the importance of 10 amino acid residues of Lcp from Streptomyces sp. K30 (LcpK30) by mutagenesis, mutein purification, and biochemical characterization. We identified several essential residues, one of which most likely represents an axial heme ligand in Lcp of Streptomyces sp. K30.
INTRODUCTION
Microbial degradation of poly(cis-1,4-isoprene) depends on rubber oxygenases that are synthesized by rubber-degrading microorganisms. Two types of rubber oxygenases are currently known: one is rubber oxygenase RoxA that has been studied in Xanthomonas sp. strain 35Y (1). RoxA is a c-type diheme-dioxygenase and has been found only in Gram-negative rubber degraders (2–4). RoxA cleaves poly(cis-1,4-isoprene) to 12-oxo-4,8-dimethyltrideca-4,8-diene-1-al (ODTD) as the major product. The structure of RoxA has been solved (5), and several residues that were involved in oxygen stabilization and active site properties have been identified in the last decade (6–8). Gram-positive rubber degraders apparently do not have a RoxA ortholog but employ another type of rubber oxygenase that had been designated latex clearing protein (Lcp) (9). Lcp was first described in Streptomyces sp. strain K30 (10), and Lcp-like sequences were found in numerous Gram-positive rubber degraders (9, 11). However, biochemical data on purified Lcps have been published only for Streptomyces sp. K30 (LcpK30) (8, 12), for Gordonia polyisoprenivorans strain VH2 (LcpVH2) (13, 14), and recently for Rhodococcus rhodochrous strain RPK1 (LcpRr) (15). Lcps are heme proteins like RoxA proteins (RoxAs) but differ from the latter in amino acid sequence, in molecule size (approximately only half the molecular mass of RoxAs), and in having a b-type cytochrome (12) instead of a c-type cytochrome. Another property for which Lcps differ from RoxAs is the type of the cleavage reaction: RoxAs cleave polyisoprene processively in an exo-type fashion and produce only one major cleavage product, the C15-oligoisoprenoid ODTD. In contrast, Lcps cleave rubber randomly (endo-type cleavage) and produce a mixture of cleavage products of different lengths. ODTD is the smallest cleavage product of the Lcp-catalyzed reaction but appears only in trace amounts in the product fraction. The majority of the cleavage products are larger and range from C20 to at least C70 oligoisoprenoids (8, 15, 16), all of which have the same keto and aldehyde end groups due to the oxidative cleavage of the polyisoprene at the double bonds.
Currently, no information is available on how Lcp catalyzes the cleavage of polyisoprene and which residues are important or essential for the catalytic reaction. We addressed this question by a bioinformatic approach that helped us to identify potentially important residues in LcpK30. Mutagenesis of identified targets and the characterization of 10 constructed and purified Lcp muteins enabled us to identify several essential residues in Lcp.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Table 1 shows the bacterial strains, plasmids, and oligonucleotides that were used in this study. Plasmid-carrying recombinant Escherichia coli strains were grown with LB medium at 22°C or 37°C in the presence of the appropriate antibiotic. Polyisoprene latex was kindly provided by Weber & Schaer (Hamburg, Germany) and was used after 3 washing steps in 0.1% (wt/vol) Nonidet P-40.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or nucleotide | Relevant characteristic(s) or sequencea | Source or reference |
|---|---|---|
| E. coli JM109 (SN3688) | Plasmid storage and expression of lcp | |
| E. coli XL1-Blue (SN4827) | Transformation strain | Stratagene |
| pUC9::strep-lcpK30 (SN5339) | Cloning vector for lcpK30, Apr | |
| p4782.1 (SN3513) | Mobilizable broad host range expression vector, Kmr | 21 |
| p4782.1::strep-lcpK30 (SN5496) | Coding sequence of strep-lcpK30 under rhamnose promoter control, Kmr | 12 |
| p4782.1::strep-lcpRr (SN5760) | Coding sequence of strep-lcpRr under rhamnose promoter control, Kmr | 15 |
| p4782.1::lcpK30 R164A (SN6034) | R164A mutein of SN5496 | 12 |
| p4782.1::lcpK30 T168A (SN6039) | T168A mutein of SN5496 | 12 |
| p4782.1::lcpK30 H198A (SN5528) | H198A mutein of SN5496 | 12 |
| p4782.1::lcpK30 H203A (SN5530) | H203A mutein of SN5496 | 12 |
| p4782.1::lcpK30 H232A (SN6035) | H232A mutein of SN5496 | 12 |
| p4782.1::lcpK30 H259A (SN6040) | H259A mutein of SN5496 | 12 |
| p4782.1::lcpK30 H266A (SN6041) | H266A mutein of SN5496 | 12 |
| p4782.1::lcpK30 R195A (SN5527) | R195A mutein of SN5496 | 12 |
| p4782.1::lcpK30 R202A (SN5529) | R202A mutein of SN5496 | 12 |
| p4782.1::lcpK30 R328A (SN6042) | R328A mutein of SN5496 | 12 |
| Fw-R164A | GACATGAAGGACGCAATCGCTAAGACCGC | |
| Rev-R164A | GCGGTCTTAGCGATTGCGTCCTTCATGTC | |
| Fw-T168A | ACCGAATCGCTAAGGCCGCACGTCTCGGT | |
| Rev-T168A | ACCGAGACGTGCGGCCTTAGCGATTCGGT | |
| Fw-H198A | ACGCATGGTGGCAGCGGCTGTTCGCC | |
| Rev-H198A | GAACAGCCGCTGCCACCATGCGTGTC | |
| Fw-H203A | GGCTGTTCGCGCACTGCTGCCGCAGT | |
| Rev-H203A | GCGGCAGCAGTGCGCGAACAGCCGCG | |
| Fw-H232A | ATGGTCACCTGGGCAAGCCTGGCCACT | |
| Rev-H232A | AGTGGCCAGGCTTGCCCAGGTGACCAT | |
| Fw-H259A | GCGGAGGCTTACCTTGCAGTGTGGCAGGT | |
| Rev-H259A | GACCTGCCACACTGCAAGGTAAGCCTCCG | |
| Fw-H266A | TGGCAGGTCAGTGCGGCAATGCTCGGAGT | |
| Rev-H266A | GACTCCGAGCATTGCCGCACTGACCTGCC | |
| Fw-R195A | AGTGAAGACAGCAATGGTGCACGCGG | |
| Rev-R195A | CGTGCACCATTGCTGTCTTCACTGCG | |
| Fw-R202A | CGCGGCTGTTGCACACCTGCTGCCGC | |
| Rev-R202A | GCAGCAGGTGTGCAACAGCCGCGTGC | |
| Fw-R328A | GGCGCGTTCTCGGCGTACACACTCGGCGG | |
| Rev-R328A | GCCGCCGAGTGTGTACGCCGAGAACGCGC | |
| pInt_F | CCCATTTTCCTGTCAGTAAC | |
| pInt_R | CTCCACGGGGAGAGCCTGAG |
Kmr, kanamycin resistance; Apr, ampicillin resistance.
Construction of lcpK30 variants.
The plasmid pUC9::strep-lcpK30 and PrimeSTAR DNA polymerase were used for a QuikChange PCR. The DNA sequences of the oligonucleotides used are given in Table 1. The PCR products were transformed to competent E. coli XL1-Blue cells via the heat shock protocol after digestion of the methylated template by DpnI. The plasmids harboring the modified lcp sequence and the vector p4782.1 were both cut with HindIII and NdeI and subjected to agarose gel electrophoresis. The band harboring the lcp variant sequence (≈1.2 kbp) and the vector (≈2.5 kbp) were purified separately and ligated and transformed into competent E. coli JM109. As a result, the lcp gene variants were cloned under the control of an l-rhamnose-dependent promoter. The lcp DNA sequences of the isolated plasmids were confirmed for each lcpK30 variant by Sanger DNA sequencing.
Bioinformatic analysis of Lcps.
The amino acid sequences of Lcps contain a domain of unknown function (DUF2236), annotated with the identifier IPR018713 in the InterPro database and PF09995 in Pfam. In June 2016, 6,598 sequences were accessible from the InterPro website that contained this domain. Of these, 5,579 were of eubacterial origin (1,005 eukaryotic sequences, only 1 archaeal sequence, and 13 unclassified sequences). The first 1,500 prokaryotic sequences were used for deeper analysis. The amino acid sequences of the three biochemically characterized Lcps contain 407 or 408 residues; therefore, we excluded sequences shorter than 250 and longer than 450 residues. This led to 1,280 sequences (*.mfa file) that were employed for a multiple-sequence alignment using the online service Clustal Omega (17). The resulting *.clustal file was visualized using Jalview (18) to identify conserved residues in this domain. A BLASTp search with the amino acid sequence of LcpK30 as the query (GenBank accession no. Q3L8N0.2) revealed 1,259 homologous sequences; 495 sequences were chosen based on identity, coverage, sequence length, and E value. A multiple-sequence alignment was performed with Clustal Omega and visualized using Jalview. The sequences of the two other biochemically characterized Lcps from R. rhodochrous RPK1 (GenBank accession no. KU140417.1; LcpRr) and G. polyisoprenivorans VH2 (GenBank accession no. ABV68923.1; LcpVH2) are provided for comparison.
Purification of LcpK30 and LcpK30 muteins.
Purification of LcpK30 was performed as described previously (12) with slight modifications: four to eight separate 600-ml LB cultures supplemented with l-rhamnose (0.1%, wt/vol) in 3-liter Erlenmeyer flasks were inoculated each with approximately 0.02 volume of a seed culture of E. coli JM109 harboring the plasmid p4782.1::lcpK30-variant that had been grown with the same medium. Cells were harvested by centrifugation after 20 to 24 h of growth at 22°C and were immediately used for protein purification. The cell pellet was resuspended in 100 mM potassium phosphate buffer (pH 7.7), containing 150 mM sodium chloride (KPN) (2 ml KPN/g cell wet weight). A soluble cell extract was prepared by two French press steps and subsequent centrifugation at 40,000 × g for 40 min. The supernatant (≈60 ml) was directly applied to a 10-ml Strep-Tactin HC gravity flow column that had been equilibrated with KPN buffer. The column was washed with at least 5 volumes of KPN buffer before Lcp was eluted by ≈30 ml of 5 mM desthiobiotin dissolved in KPN. Lcp-containing fractions were combined, desalted by passage through a G25 Sephadex (26/160) HiPrep desalting column (53-ml bed volume) that had been equilibrated with 1 mM potassium phosphate (KP) buffer (pH 7.0) and subsequently concentrated to 1 to 2 ml via ultrafiltration (10-kDa cutoff). Combined LcpK30-containing fractions were concentrated to 1 to 1.5 ml via ultrafiltration (10-kDa cutoff). Aliquots of the purified LcpK30 muteins were stored on ice for up to 1 week for the stable muteins or shock-frozen with liquid nitrogen and stored at −70°C. Activity and other biochemical data of instable muteins (R195A and R202A) were determined on the same day of purification.
Assay of Lcp activity.
A high-performance liquid chromatography (HPLC)-based assay to separate and quantify Lcp-derived polyisoprene degradation products was used for most routine assays: poly(cis-1,4-isoprene) latex was diluted with 100 mM KP buffer (pH 7) to 0.2% (assay volume, 0.7 ml) and incubated in the presence of the purified Lcp mutein for 2 h at 23°C if not stated otherwise. The products were extracted with 1 ml ethyl acetate (in a 2-ml Eppendorf tube), dried, and dissolved in 100 μl methanol. Aliquots were applied to an RP8 HPLC column (12 by 4 mm, 5-μm particle size, 0.7 ml/min) with water (A) and methanol (B) as mobile phases. The concentration of methanol was increased from 50% (vol/vol) to 100% (vol/vol) within 15 min; products were detected at 210 nm. The C35 product peak (at ≈23 min) was used for quantification and compared to a control without enzyme. Alternatively, the activity of LcpK30 variants was assayed by the determination of the rate of oxygen consumption in an OXY-4 mini apparatus (PreSens, Regensburg, Germany) as described previously (8). Triplicates and controls without enzyme or with heat-inactivated Lcp were recorded simultaneously.
Circular dichroism spectroscopy.
Circular dichroism (CD) spectra were recorded with a J-815 CD spectrophotometer (Jasco, Gross-Umstadt, Germany). Measurements were performed in 0.2-mm (80-μl volume) cuvettes for highly concentrated samples or in 1.0-mm cuvettes (350-μl volume) for less concentrated protein solutions in 1 mM KP buffer (pH 7.0). Spectra were recorded in a range from 185 to 260 nm with standard sensitivity and a bandwidth of 1 nm. Spectra without protein (KP buffer only) were subtracted from the spectrum of the protein solution. Thirty scans were collected and averaged. The melting curve was measured at the CD minimum with an increasing temperature of 1°C/min from 20 to 80°C for selected muteins.
Other techniques.
The concentration of protein solutions was determined by the bicinchoninic acid (BCA) method. The concentrations of purified heme-containing proteins were also determined from the molar extinction coefficient of LcpK30 (ε412 = 8.0 × 104 M−1 cm−1). Proteins were separated by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate (SDS-PAGE) under reducing (2-mercaptoethanol) conditions. SDS-PAGE gels were stained with silver.
RESULTS AND DISCUSSION
Bioinformatic analysis of the domain of unknown function (DUF2236) and Lcps.
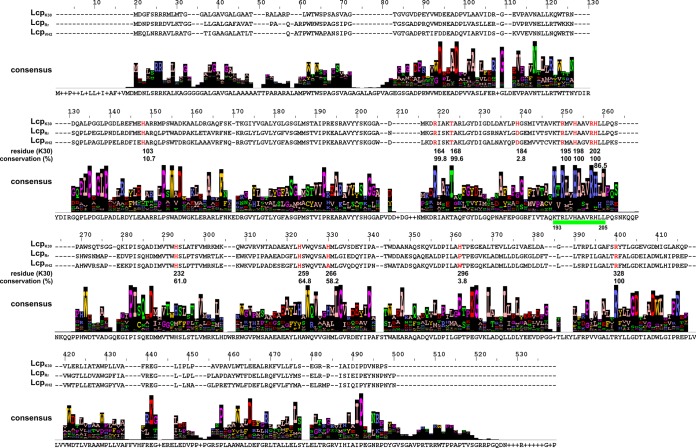
All Lcps biochemically characterized so far (12, 14, 15) have a common domain of unknown function (DUF2236) that covers roughly half of the entire amino acid sequence. Currently, more than 6,000 DUF2236-containing sequences can be retrieved from databases, most of which refer to a prokaryotic origin (IPR018713 [InterPro] and PF09995 [Pfam]). We chose 1,280 of those DUF2236 sequences based on their similarities among each other and their sequence lengths (the identifiers of the sequences used are listed in the supplemental material). We performed a multiple-sequence alignment to analyze the conservation of the amino acids (see Fig. S1 in the supplemental material). Only three residues were conserved in more than 80% of all sequences. These were R164 (97.9%, numbering according to LcpK30), T168 (84.7%, 13.4% serine), and H198 (98.7%). We designated R164, T168, and H198 DUF2236-specific residues. Many of the 1,280 sequences were annotated as putative Lcps. However, hypothetical proteins with different supposed functional annotations such as proteases or histidine kinases were also present among the DUF2236 sequences, and this indicated that the DUF2236 domain is not necessarily restricted to Lcps. A BLASTp search with the LcpK30 amino acid sequence was performed and led to related sequences (for details, see Materials and Methods) that were aligned (Fig. 1). It is evident that the three conserved DUF2236 residues (R164, T168, and H198) were highly conserved in the 495 Lcp homologues by 98.8, 99.6, and 100%, respectively. We conclude that these residues are of high importance for the function of Lcps and presumably for DUF2236 proteins in general. An additional 32 amino acids, beside the 3 above-mentioned conserved arginine, threonine, and histidine residues, were also highly conserved (>90%) in the 495 Lcp homologues (Table 2).
FIG 1.
Multisequence alignment of 495 amino acid sequences homologous to LcpK30. A BLASTp search with the sequence of LcpK30 as query revealed many homologous sequences of which 495 were chosen for this alignment, considering sequence coverage, length, and similarity. The sequences of the biochemically characterized Lcps are provided, and the residue numbering refers to LcpK30. The values of conservation (as percentages) for the residues chosen in this study (highlighted in red) are given below. A 13-residue-long highly conserved region is indicated by a green bar. The degree of conservation of a specific residue position is indicated by the height of the respective column.
TABLE 2.
Conserved residues identified in the multiple-sequence alignment of 495 Lcp homologous proteins and histidine residues investigated in this studya
| Residue | No. | Conservation (%) |
|---|---|---|
| G | 269 | 100 |
| H | 198 | 100, DUF |
| P | 108 | 100 |
| R | 195 | 100 |
| R | 202 | 100 |
| R | 328 | 100 |
| R | 164 | 99.8, DUF |
| P | 221 | 99.8 |
| G | 300 | 99.8 |
| W | 261 | 99.8 |
| N | 76 | 99.6 |
| T | 168 | 99.6, DUF |
| W | 110 | 99.6 |
| P | 94 | 99.2 |
| P | 401 | 99.2 |
| D | 56 | 99.0 |
| D | 60 | 99.0 |
| P | 90 | 99.0 |
| D | 226 | 98.6 |
| L | 268 | 98.0 |
| L | 307 | 98.0 |
| P | 276 | 98.0 |
| D | 112 | 97.8 |
| K | 193 | 97.6 |
| W | 211 | 96.6 |
| E | 299 | 96.4 |
| L | 303 | 96.0 |
| I | 222 | 95.8 |
| P | 146 | 95.4 |
| A | 282 | 94.7 |
| E | 363 | 94.7 |
| A | 199 | 93.3 |
| L | 205 | 92.7 |
| L | 93 | 92.5 |
| F | 122 | 91.5 |
| H | 203 | 86.5 |
| H | 232 | 61.0 |
| H | 259 | 64.8 |
| H | 266 | 58.2 |
| H | 103 | 10.7 |
| H | 296 | 3.8 |
| H | 184 | 2.8 |
The residues are ordered according to the highest conservation; only residues conserved >90% are listed except for the histidine residues investigated in this study. Alanine muteins of the respective residues that were purified in this study are printed with bold letters. DUF indicates that the respective residue corresponds to one of the three highly conserved residues in the amino acid sequences of proteins with a DUF2236 domain.
Among the 32 highly conserved residues, there were 3 arginine residues (R195, R202, R328), 1 lysine residue (K193), 4 small residues (G269, G300, A199, A282), and 7 acidic/amide residues (D56, D60, N76, D112, D226, E299, E363). Notably, 7 proline residues (P90, P94, P108, P146, P221, P276, P401) that might function as helix breakers in the structure of the proteins were also identified. Concerning conserved aromatic residues, only 1 phenylalanine (F122) and 3 tryptophans (W110, W211, W261) were identified. Five leucines (L93, L205, L268, L303, L307) and 1 isoleucine (I222) complete the list of conserved residues (Table 2). Another outcome of our bioinformatic analysis was the identification of a 13-residue-long highly conserved region in the primary amino acid sequence between K193 and L205 (in LcpK30) (Fig. 1). Three of the 100% conserved residues were located in this part (R195, H198, R202) and suggest that this region is of high importance for the functionality of Lcps.
All three conserved DUF2236 residues are essential for the activity of LcpK30.
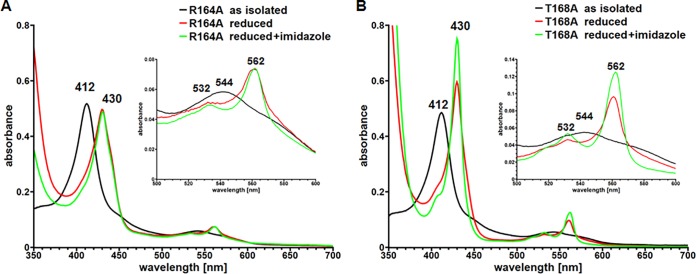
To get experimental evidence for the functional importance of the three highly conserved DUF2236 residues (R164, T168, and H198), we substituted all three residues individually by alanine. Wild-type LcpK30 and the three LcpK30 muteins were each expressed in E. coli, purified (Fig. 2), and biochemically characterized. For the R164A mutein, 10 to 15 mg was obtained in two separate purifications from a 4.8-liter culture, amounting to roughly one-third of the yield achieved for the wild type. In the case of T168A, the yield was 15 to 20 mg. Concentrated solutions of both muteins (R164A and T168A) were red (as the wild-type protein) and showed a typical heme spectrum very similar to that of the wild type (absorption maxima at 412 nm [Soret, γ] and 544 nm [β-band] in the as-isolated state). In the dithionite-reduced state, we observed the same absorption bands for the R164A and T168A muteins as for the wild-type protein with maxima at 430 nm (Soret), 532 nm (β-band), and 562 nm (α-band). However, no increase in the 430-nm Soret band relative to the Soret band in the as-isolated (oxidized) state was observed for the R164A mutein and the 532-nm β-band was not as pronounced as those observed for the wild type and the T168A mutein. This indicated that slight structural changes had occurred in the vicinity of the heme for the R164A mutein (Fig. 3). Both muteins were stable during prolonged storage on ice and revealed a CD spectrum similar to that of wild-type LcpK30. These data confirmed that the structures of the Lcp muteins were comparable to that of the wild type. Surprisingly, no polyisoprene-cleaving activity was detected for either mutein. However, by extension of the incubation time of the activity assay from 2 h to 16 h and by the increase of the amount of mutein from 4 μg to 50 μg, we detected small amounts of polyisoprene cleavage products for the T168A mutein. These corresponded to ≈2% of the activity that was determined for wild-type LcpK30. In the case of R164A, the increase in incubation time and increase in the amount of enzyme did not result in the detection of any activity. We conclude that T168 and R164 represent catalytically important or essential residues in LcpK30. Consequently, this presumably counts for all homologous Lcps due to the high conservation of these residues.
FIG 2.
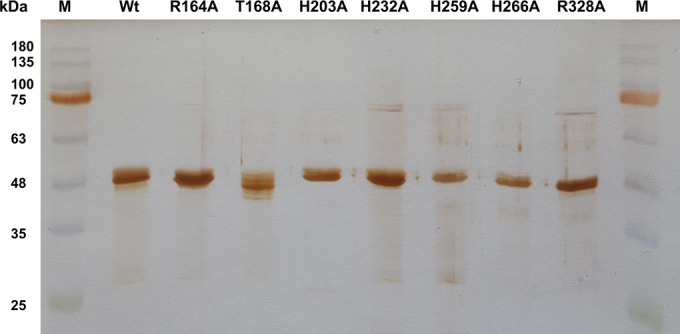
SDS-PAGE of purified LcpK30 muteins. Molecular mass standards (M) with kilodalton values indicated are shown in the left-most and right-most lanes. The type of mutein is indicated in the top line. All proteins had been stored frozen (−20°C) before electrophoresis. For the T168A mutein, the protein band appeared inhomogeneous. This could be attributed to the storage period and storage condition. The freshly purified T168A mutein appeared homogeneous. Wt, wild type.
FIG 3.
UV-vis spectra of purified LcpK30 R164A and LcpK30 T168A. UV-vis spectra of purified R164A (A) and T168A (B) were recorded in the as-isolated state (black lines), in the dithionite-reduced state (red lines), and in the dithionite-reduced state to which 10 mM imidazole had been added (green lines). The Q band region between 500 and 600 nm is shown in an enlarged form in the insets. The wavelengths of the absorption maxima are indicated. Notably, no increase in the Soret band was observed for the R164A mutein, indicating differences in the vicinity of the heme. A significant increase in the absorption of the α-band at 562 nm was observed for the T168A mutein after addition of imidazole to the reduced protein, similar to LcpRr (15), indicating a change from an open to a closed state. For UV-vis spectra of the LcpK30 wild type see Fig. S2 in the supplemental material.
The yield of the LcpK30 H198A mutein was approximately 3 mg (from a 4.8-liter culture), and this accounted for less than 10% of the yield determined for purified wild-type protein. LcpK30 H198A was moderately stable (only some degradation bands became visible by SDS-PAGE analysis during prolonged storage of the mutein) and was colorless. The absence of a typical heme UV-visible (UV-vis) spectrum confirmed that the LcpK30 H198A mutein did not contain a heme group. Accordingly, no oxidative polyisoprene-cleaving activity was detected in the two activity assays (product and oxygen consumption assay). We conclude that the third DUF-2236-specific residue (H198) is also essential for LcpK30 function. Immediately after purification, we recorded a CD spectrum of LcpK30 H198A that showed the same characteristics as that of the wild type and thus confirmed the correct folding of the mutein. The absence of an incorporated heme group in an otherwise moderately stable apoprotein indicated that H198 likely participates in the binding of the heme group. It is well known for cytochromes that the heme groups have at least one axial histidine ligand (19). The high degree of conservation of a histidine residue at this position (Fig. 1) is in agreement with the assumption that H198 most likely represents an axial heme ligand in LcpK30.
LcpK30 presumably has no histidine residue as a second axial ligand.
Previous analysis in our group had shown that wild-type LcpK30 as-isolated is present in a closed state and presumably has two axial amino acids as ligands (12, 15). This assumption was based on experiments in which the changes in the UV-vis spectra of wild-type LcpK30 were recorded upon the addition of typical heme ligands such as imidazole. The absence of any changes in the UV-vis spectrum upon the addition of imidazole (see Fig. S2 in the supplemental material) suggested that the heme group in LcpK30 is coordinated by two axial ligands that prevent binding of the free solvent imidazole to the heme group. We concluded that wild-type LcpK30 most likely is present in a closed conformation compared to LcpRr, which was resting in an open, accessible for ligands, state (15). The presence of a dioxygen molecule as an axial ligand as had been determined for the rubber oxygenase RoxA of Xanthomonas sp. 35Y (5) was excluded because the heme group of wild-type LcpK30 was present in the oxidized (Fe3+) state. This assumption was supported by the inability of LcpK30 to bind carbon monoxide. A heme-bound dioxygen molecule would require a reduced (Fe2+) state, and a reduced heme group (Fe2+) would be able to bind carbon monoxide as is the case for RoxA (5). These considerations suggest that both axial ligands in LcpK30 are amino acids. To identify possible residues that might serve that function, we used the search tool of the Heme Protein Database (20). The most frequent conserved axial ligands were histidine residues (80 cases) followed by a bound dioxygen molecule (41 cases), bound methionine residues (5 cases), and cyanide molecules (5 cases). We focused on histidine residues that might represent axial heme ligands. One axial ligand (H198) was already identified as pointed out above. The amino acid sequence of LcpK30 has only seven other histidine residues in addition to H198 (H103, H184, H203, H232, H259, H266, H296) (Table 2). Out of these, three residues are not conserved at all in sequences homologous to LcpK30 (H103, 10.7% conservation; H296, 3.8% conservation; and H184, 2.8% conservation) and therefore are unlikely to represent an axial heme ligand. The four remaining histidine residues are conserved in all biochemically characterized Lcps and in more than 58% of all Lcp homologous sequences (Fig. 1; Table 2) and therefore might represent important residues. We substituted all four moderately conserved histidine residues individually by alanine and purified the respective LcpK30 muteins (Fig. 2). All four LcpK30 muteins (H203A, H232A, H259A, H266A) were stably expressed, were red, and showed specific activities very similar to those determined for wild-type LcpK30 (for details, see Table 3). We concluded that none of the four histidine residues is essential for activity. The activity and the product spectra of the oligoisoprenoids produced were not significantly changed compared to those of the wild type (see Fig. S3 and S4 in the supplemental material). UV-vis spectroscopic studies of all four muteins (in the as-isolated state, in the dithionite-reduced state, and in the reduced state to which 10 mM imidazole was added) (see Fig. S2 in the supplemental material) also showed no substantial differences from wild-type LcpK30, indicating the coordination of the second axial ligand to the heme center. Thus, the involvement of these four histidines as a second axial ligand can be excluded. The LcpK30 amino acid sequence has 14 methionine residues which are, however, not or only moderately conserved in the 495 Lcp-like sequences. In general, it is difficult to identify the second axial ligand of LcpK30 due to a presumably lower conservation rate within the Lcp family, as was shown for the missing second ligand in LcpRr (15). At present, the nature of the second axial residue remains unknown.
TABLE 3.
Properties of RoxA and of Lcp proteins
| Characteristic | Data according to source/proteina |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xanth. sp./RoxAb | G. p. VH2/LcpVH2c | R. r. RPK1/LcpRrd | Wt/LcpK30 | R164A/LcpK30 | T168A/LcpK30 | H198A/LcpK30 | H203A/LcpK30 | H232A/LcpK30 | H259A/LcpK30 | H266A/LcpK30 | R195A/LcpK30 | R202A/LcpK30 | R328A/LcpK30 | |
| Type | Wt | DUF2236 residue | DUF2236 residue | DUF2236 heme ligand | Wt like | Wt like | Wt like | Wt like | Instable mutein | Instable mutein | Wt like | |||
| Color | Red | Orange | Brown | Red | Red | Red | Colorless | Red | Red | Red | Red | Colorless | Colorless | Red |
| Conservation (%)e | 99.8 | 99.6 | 100 | 86.5 | 61.0 | 64.8 | 58.2 | 100 | 100 | 100 | ||||
| Yield (purified) (mg/liter LB medium) | 3 | 10 | 3 | 4 | 0.5 | 1 | 2 | 0.3 | 4 | 0.1 | 0.05 | 0.3 | ||
| UV/vis spectra as-isolated (nm) | ||||||||||||||
| Soret band | 407 | 407 | 412 | 412 | 412 | 412 | 412 | 412 | 412 | 411 | 412 | |||
| Q band (β) | 534 | 535 | 544 | 544 | 544 | 544 | 544 | 544 | 544 | 544 | ||||
| 645 | ||||||||||||||
| Dithionite reduced | ||||||||||||||
| Soret band | 417 | 428 no increase | 430 increased | 430 no increase | 430 increased | 430 no increase | 430 no increase | 430 no increase | 430 no increase | 430 no increase | ||||
| Q band (β) | 521 | 531 | 532 | 532 | 532 | 532 | 532 | 532 | 532 | 532 | ||||
| Q band (α) | 549/553 | 560 | 562 | 562 | 561 | 562 | 562 | 562 | 561 | 562 | ||||
| Reduced + imidazole | 428 increased | No effect | No effect | 430 increased | Wt like | Wt like | Wt like | Wt like | Wt like | |||||
| Activity | 532 + 561 increase | 532 + 562 increase | ||||||||||||
| HPLC product assay | ODTD | Typical pattern | Typical pattern | Typical pattern Wt | Inactive | Residual activity | Inactive | Wt like | Wt like | Wt like | Wt like | Inactive | Residual activity | Wt like |
| Oxygen consumption (U/mg) (22°C) | 1.3 | 0.9 | 1.5 | <0.1 | <0.1 | <0.1 | Wt like | Wt like | Wt like | Wt like | Inactive <0.1 | Inactive <0.1 | Wt like | |
| CD spectra | Wt | Wt like | Wt like | Wt like | Wt like | Wt like | ||||||||
| Melting point | 54.3 | 61.5 | 61.6 | |||||||||||
Empty space indicates that the value has not been determined. Xanth. sp., Xanthomonas sp. 35Y; G. p. VH2, Gordonia polyisoprenivorans strain VH2; R. r. RPK1, Rhodococcus rhodochrous strain RPK1.
Data from reference 5.
Data from reference 14.
Data from reference 15.
In an alignment of 495 LcpK30 homologues.
In a recent publication on the properties of purified Lcp of G. polyisoprenivorans (LcpVH2), substantial amounts of copper were detected, and the authors speculated that the histidine residues corresponding to H198, H203, and H232 in LcpK30 potentially might participate in the coordination of a copper ion (14). A function of H198, H203, and H232 in the coordination of a copper ion is unlikely for LcpK30: first, only substoichiometric trace amounts of copper were detected in purified LcpK30 (12); second, the finding in this study that the LcpK30-H198A mutein is colorless and has lost its heme cofactor suggested that H198 probably represents an axial heme ligand; and third, the determination of wild-type levels of activity for the purified LcpK30-H203A and LcpK30-H232A muteins provides further evidence that a copper ion is not coordinated by these residues.
Functional analysis of conserved arginine residues in LcpK30.
The amino acid sequence of LcpK30 harbors several, highly conserved arginine residues. Besides the DUF2236-conserved residue R164 (see above), the arginine residues R195, R202, and R328 were conserved in all 495 Lcp homologous sequences. R195 and R202 are part of the 13-amino-acid-long sequence in which all residues are highly conserved in Lcp homologous sequences (Fig. 1). Since arginine residues are positively charged, they are probably not involved in the binding of the hydrophobic polyisoprene substrate and should have other functions. To investigate the importance of the strictly conserved arginine residues, we constructed lcpK30 mutants in which the codons for R195, R202, and R328 were substituted by alanine codons. All three muteins were individually expressed and each mutein was purified. The R195A and R202A LcpK30 muteins were rather unstable. While samples that were taken immediately from the purified muteins of the eluate fraction from the Strep-Tactin column appeared homogeneous in SDS-PAGE analysis, a large fraction of the protein moiety subsequently precipitated and became degraded during the concentration and diafiltration process (buffer exchange from 100 mM KP buffer with 150 mM NaCl for 1 mM KP buffer without salt). Therefore, only low yields of 0.2 mg purified protein in case of LcpK30-R202A and 1 mg LcpK30-R195A were obtained in contrast to 40 to 50 mg for wild-type LcpK30. Remarkably, the LcpK30 muteins R195A and R202A were (almost) colorless and, accordingly, were inactive in the oxygen consumption activity assay. In the UV-vis spectrum of R202A, a very slight absorption at ≈411 nm was detected immediately after purification, indicating the presence of trace amounts of heme-containing mutein (see Fig. S2 in the supplemental material). We incubated 50 μg of R202A in 1 ml of assay buffer with polyisoprene latex immediately after the purification and were able to determine residual activity of 2% compared to that for the wild type and with the same pattern of cleavage products (see Fig. S4 in the supplemental material). This indicated that immediately after purification, a minor fraction of the LcpK30 mutein with heme incorporated and with correct folding was present but that a substantial degradation of the mutein rapidly occurred. We conclude that the two residues (R195 and R202) are essential for the stability of the protein, and the data confirm the importance of the two arginine residues in the conserved 13-amino-acid-long sequence between K193 and L205 in LcpK30. The two arginines might be part of salt bridges generally stabilizing the protein structure by binding to one of the many highly conserved acidic glutamate or aspartate residues (D56 [99% conservation], D60 [99%], D112 [97.8%], D226 [98.6%], E299 [96.4%], and E363 [94.7%]).
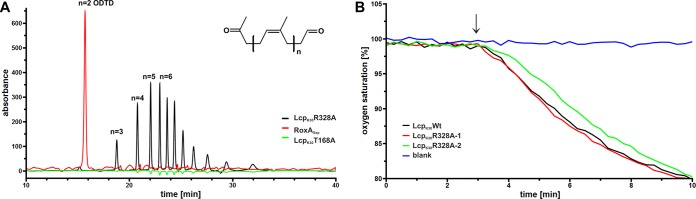
R328 was strictly conserved in Lcp homologous sequences but was not part of the highly conserved region. Notably, the purified mutein R328A was red and stable, contained the heme group, and showed oxidative polyisoprene cleavage activity comparable to that of the wild-type protein (Fig. 4). Apparently, the position at 328 in the LcpK30 amino acid sequence is not essential for activity or stability and the reason for the high degree of conservation at this position remains unclear.
FIG 4.
Activities of LcpK30 muteins. (A) Activity of purified LcpK30 variants was determined by HPLC-based analysis of the cleavage products. For Lcp R328A, the typical wild-type pattern of oligoisoprenoids varying in the number of isoprene units (n) was observed (black line). For comparison, purified RoxA from Xanthomonas sp. 35Y producing 12-oxo-4,8-dimethyltrideca-4,8-diene-1-al (ODTD) (n = 2) as sole major degradation product was also used (red line). For the wild type and most Lcp muteins, 4 μg of purified Lcp and a 2-h assay time were applied. For LcpK30 T168A (green line), an increased amount of enzyme (50 μg) and a longer incubation time (16 h) were used before the cleavage products were extracted with ethyl acetate and analyzed by HPLC. Note the very small amounts of degradation products with the same retention times and pattern produced by the T168A mutein. For data for the LcpK30 wild type and other Lcp muteins, see Fig. S4 in the supplemental material. (B) Assay of Lcp activity via the monitoring of oxygen consumption. After linearity of the oxygen consumption (3 min), the respective enzymes were added (arrow), initiating a decrease in oxygen saturation due to polyisoprene cleavage. Two parallel runs were recorded for the R328A mutein (red and green lines). Blank refers to a control without enzyme. For data for the other Lcp muteins see Fig. S3 in the supplemental material.
Spectroscopic characterization of LcpK30 muteins.
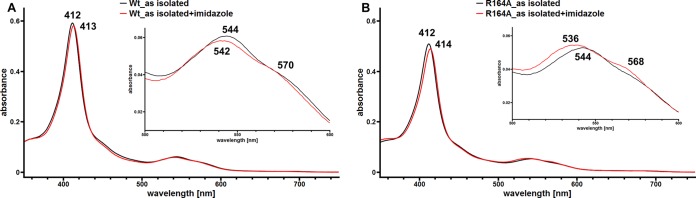
Mutational analysis of the LcpK30 muteins with changes in the three DUF2236-specific residues (R164A, T168A, and H198A) revealed that all three residues are essential for Lcp activity (see above). Further spectroscopic investigation of the H198A mutein was not possible because this mutein was colorless and had no heme incorporated. We therefore concentrated on the investigation of the spectroscopic properties of the R164A and T168A muteins. Both residues are located in the 13-residue-long sequence that is conserved to a high degree in the 495 Lcp-like sequences. Figure 3 shows the UV-vis spectra of both LcpK30 muteins in the as-isolated state, in the dithionite-reduced state, and in the reduced state in the presence of 10 mM added imidazole in comparison to those for wild-type LcpK30. Both LcpK30 variants showed comparable spectra in the as-isolated state that are typical for heme proteins in the oxidized form. However, reduction of R164A by dithionite did not result in increased absorption of the Soret band at 430 nm relative to 412 nm in the as-isolated state as was observed for the wild type (see Fig. S2 in the supplemental material). The absorption of the Q bands at 532 nm and 562 nm was not as pronounced either, indicating minor differences in the vicinity of the heme. Addition of imidazole to the reduced LcpK30 R164A mutein did not result in increased absorption, indicating a closed state of the mutein as observed for wild-type LcpK30. A very minor effect (slight increase at 568 nm in the UV-vis spectrum) was observed when imidazole was added to the R164A protein in the as-isolated state (Fig. 5). This effect was less obvious for the wild-type protein. The meaning of this minor effect is unclear.
FIG 5.
UV-vis spectra of LcpK30 wild type (A) and the R164A mutein (B). UV-vis spectra of purified Lcp in the as-isolated state (black line) and in the as-isolated state to which 10 mM imidazole had been added (red line). The region between 500 and 600 nm is shown in an enlarged form in the insets. The wavelengths of the absorption maxima are indicated. For LcpK30 R164A, a weak band around 568 nm occurred that was less pronounced (at 570 nm) for the LcpK30 wild type or for other heme-containing muteins.
The dithionite-reduced mutein T168A revealed increased absorption of the Soret band (at 430 nm) relative to that of the 412-nm Soret band of the oxidized protein (Fig. 3). The 430-nm (and 562-nm) bands further increased when imidazole was added to the dithionite-reduced protein. These changes in the UV-vis spectra were not observed for the wild-type protein or the R164A mutein (Fig. 3; see also Fig. S2 in the supplemental material) and indicated that the heme group of the reduced T168A mutein was present in an open form, accessible for imidazole. The increases in the absorption at 430 nm and 562 nm of the T168A mutein in the presence of dithionite and imidazole were very reminiscent of that of the Lcp protein of R. rhodochrous RPK1 (15). The latter also showed increased absorption of the Soret band and α-band in the presence of dithionite and imidazole, and it was assumed that the LcpRr protein in the as-isolated state is present in an open conformation in contrast to LcpK30, which most likely is present in a closed form (12, 15). Our data indicate that the residue T168 is located close to the active center and to the heme group so that the exchange of an CH2OH group for a methyl group can switch the enzyme from a closed (imidazole not accessible) to an open (imidazole-accessible) form.
The spectroscopic characterizations of all other heme-containing (red) LcpK30 muteins in this study (H203A, H232A, H259A, H266A, R328A) showed no detectable differences in the UV-vis spectra of the as-isolated, the dithionite-reduced, and the dithionite- and imidazole-treated proteins (Table 3; see also Fig. S2 in the supplemental material). These data are in agreement with the wild-type activities of these muteins and confirm our assumption that these residues are not part of the active center and are not otherwise essential for activity or structure.
In summary, we have experimentally verified that the three DUF2236 domain-specific residues (R164, T168, and H198) and two conserved arginines (R195 and R202) are essential for activity, stability, and/or incorporation of the heme cofactor in LcpK30. On the basis of the high degree of conservation of these residues among Lcp and DUF2236 domain-containing proteins, we predict that these residues will be essential also for other Lcps and might likely be of importance for other DUF2236 domain-containing proteins.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft.
We thank Weber & Schaer for providing polyisoprene and the Institute of Biochemistry of the University of Stuttgart for access to CD spectroscopy.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02176-16.
REFERENCES
- 1.Tsuchii A, Takeda K. 1990. Rubber-degrading enzyme from a bacterial culture. Appl Environ Microbiol 56:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braaz R, Fischer P, Jendrossek D. 2004. Novel type of heme-dependent oxygenase catalyzes oxidative cleavage of rubber (poly-cis-1,4-isoprene). Appl Environ Microbiol 70:7388–7395. doi: 10.1128/AEM.70.12.7388-7395.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braaz R, Armbruster W, Jendrossek D. 2005. Heme-dependent rubber oxygenase RoxA of Xanthomonas sp. cleaves the carbon backbone of poly(cis-1,4-isoprene) by a dioxygenase mechanism. Appl Environ Microbiol 71:2473–2478. doi: 10.1128/AEM.71.5.2473-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birke J, Röther W, Schmitt G, Jendrossek D. 2013. Functional identification of rubber oxygenase (RoxA) in soil and marine myxobacteria. Appl Environ Microbiol 79:6391–6399. doi: 10.1128/AEM.02194-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seidel J, Schmitt G, Hoffmann M, Jendrossek D, Einsle O. 2013. Structure of the processive rubber oxygenase RoxA from Xanthomonas sp. Proc Natl Acad Sci U S A 110:13833–13838. doi: 10.1073/pnas.1305560110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitt G, Seiffert G, Kroneck PMH, Braaz R, Jendrossek D. 2010. Spectroscopic properties of rubber oxygenase RoxA from Xanthomonas sp., a new type of dihaem dioxygenase. Microbiology 156:2537–2548. doi: 10.1099/mic.0.038992-0. [DOI] [PubMed] [Google Scholar]
- 7.Birke J, Hambsch N, Schmitt G, Altenbuchner J, Jendrossek D. 2012. Phe317 is essential for rubber oxygenase RoxA activity. Appl Environ Microbiol 78:7876–7883. doi: 10.1128/AEM.02385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birke J, Jendrossek D. 2014. Rubber oxygenase (RoxA) and latex clearing protein (Lcp) cleave rubber to different products and use different cleavage mechanisms. Appl Environ Microbiol 80:5012–5020. doi: 10.1128/AEM.01271-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yikmis M, Steinbüchel A. 2012. Historical and recent achievements in the field of microbial degradation of natural and synthetic rubber. Appl Environ Microbiol 78:4543–4551. doi: 10.1128/AEM.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose K, Tenberge KB, Steinbüchel A. 2005. Identification and characterization of genes from Streptomyces sp. strain K30 responsible for clear zone formation on natural rubber latex and poly(cis-1,4-isoprene) rubber degradation. Biomacromolecules 6:180–188. doi: 10.1021/bm0496110. [DOI] [PubMed] [Google Scholar]
- 11.Nanthini J, Chia K-H, Thottathil GP, Taylor TD, Kondo S, Najimudin N, Baybayan P, Singh S, Sudesh K. 2015. Complete genome sequence of Streptomyces sp. strain CFMR 7, a natural rubber degrading actinomycete isolated from Penang, Malaysia. J Biotechnol 214:47–48. doi: 10.1016/j.jbiotec.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Birke J, Röther W, Jendrossek D. 2015. Latex clearing protein (Lcp) of Streptomyces sp. strain K30 is a b-type cytochrome and differs from rubber oxygenase A (RoxA) in its biophysical properties. Appl Environ Microbiol 81:3793–3799. doi: 10.1128/AEM.00275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiessl S, Schuldes J, Thürmer A, Halbsguth T, Broker D, Angelov A, Liebl W, Daniel R, Steinbüchel A. 2012. Involvement of two latex-clearing proteins during rubber degradation and insights into the subsequent degradation pathway revealed by the genome sequence of Gordonia polyisoprenivorans strain VH2. Appl Environ Microbiol 78:2874–2887. doi: 10.1128/AEM.07969-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiessl S, Böse D, Oetermann S, Eggers J, Pietruszka J, Steinbüchel A. 2014. Latex clearing protein-an oxygenase cleaving poly(cis-1,4-isoprene) rubber at the cis double bonds. Appl Environ Microbiol 80:5231–5240. doi: 10.1128/AEM.01502-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watcharakul S, Röther W, Birke J, Umsakul K, Hodgson B, Jendrossek D. 2016. Biochemical and spectroscopic characterization of purified Latex Clearing Protein (Lcp) from newly isolated rubber degrading Rhodococcus rhodochrous strain RPK1 reveals novel properties of Lcp. BMC Microbiol 16:92. doi: 10.1186/s12866-016-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim EMA, Arenskotter M, Luftmann H, Steinbüchel A. 2006. Identification of poly(cis-1,4-isoprene) degradation intermediates during growth of moderately thermophilic actinomycetes on rubber and cloning of a functional lcp homologue from Nocardia farcinica strain E1. Appl Environ Microbiol 72:3375–3382. doi: 10.1128/AEM.72.5.3375-3382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. 2009. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wittenberg JB, Wittenberg BA, Peisach J, Blumberg WE. 1970. On the state of the iron and the nature of the ligand in oxyhemoglobin. Proc Natl Acad Sci U S A 67:1846–1853. doi: 10.1073/pnas.67.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reedy CJ, Elvekrog MM, Gibney BR. 2008. Development of a heme protein structure-electrochemical function database. Nucleic Acids Res 36:D307−D313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altenbuchner J, Viell P, Pelletier I. 1992. Positive selection vectors based on palindromic DNA sequences. Methods Enzymol 216:457–466. doi: 10.1016/0076-6879(92)16042-I. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.