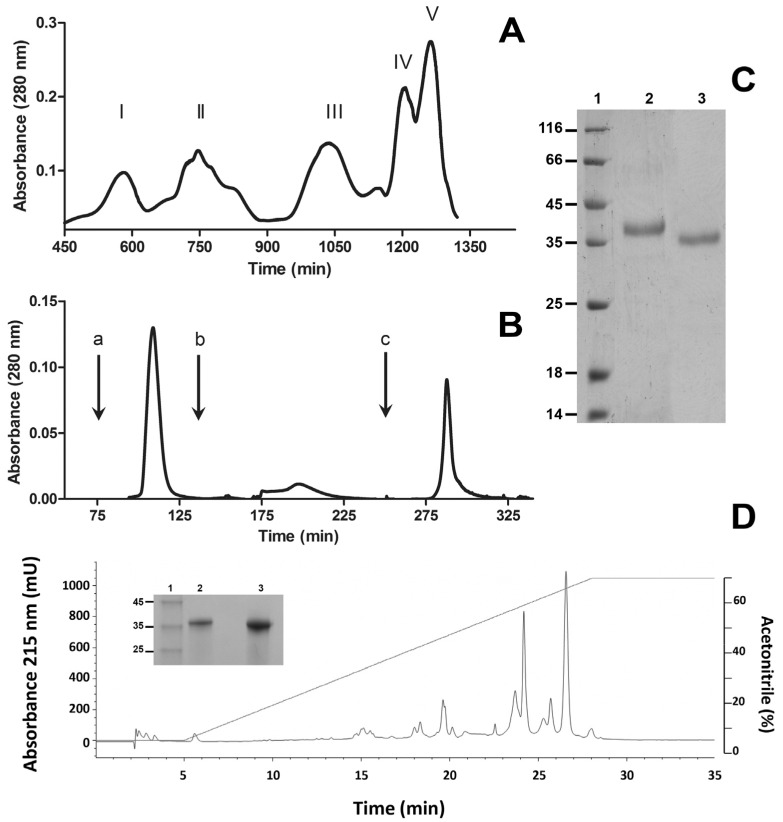
Figure 1.
Isolation of BlatPII from B. lateralis venom. Venom was fractionated by ion-exchange chromatography on diethylaminoethyl (DEAE)-Sepharose (A); and hydrophobic interaction chromatography on phenyl sepharose (B); as described in the Materials and Methods. Arrows inserted in (B) correspond to the addition of 100 mL of 0.01 M phosphate buffer, pH 7.8, containing 1 M NaCl (a); 100 mL of 0.01 M phosphate buffer, pH 7.8 (b); and 100 mL of deionized water (c). Fraction II from the hydrophobic interaction chromatography is a SVMP devoid of hemorrhagic activity and was named BlatPII. (C) SDS-PAGE of the purified protein under reducing (Lane 2) and non-reducing (Lane 3) conditions. (D) BlatPII was separated into two main peaks by RP-HPLC. Red line corresponds to acetonitrile gradient (see the text for details). SDS-PAGE under reducing conditions of Peak 1 (Lane 2) and Peak 2 (Lane 3) is shown in the insert of (D). Lane 1 in gels of (C) and the insert of (D) corresponds to molecular mass standards (kDa).