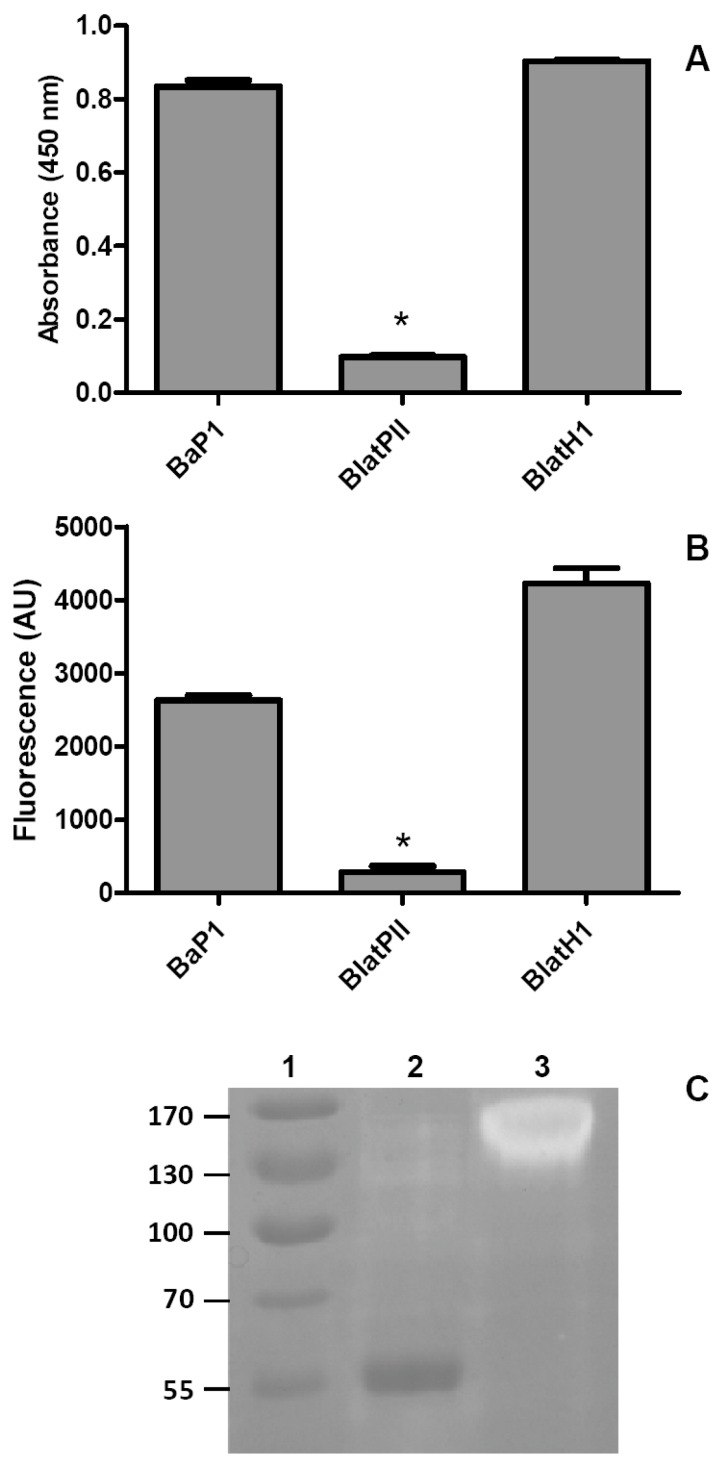
Figure 3.
Evaluation of the proteolytic activity of BlatPII and other SVMPs on azocasein and gelatin. (A) BlatPII (5.5 µM) was incubated with azocasein (see the Materials and Methods for details). For comparison, an equimolar concentration of PI SVMP BaP1, from the venom of Bothrops asper, and of the hemorrhagic PII SVMP BlatH1, from the venom of Bothriechis lateralis, were also tested. PBS was used as a negative control; (B) Quantification of the gelatinolytic activity of the SVMP by a fluorescent commercial kit (EnzCheck® protocol Gelatinase/Collagenase Assay Kit, Molecular Probes, Life Technologies, Eugene, OR, USA). One-point-six micrograms of BlatPII were incubated with 20 µg of the fluorescent gelatin; equimolar quantities of BaP1 and BlatH1 were used as positive controls; samples were incubated at room temperature for 6 h. Fluorescence intensity was measured in the BioTek Synergy HT microplate reader using the absorption filter at 495 nm and the emission filter at 515 nm. * p < 0.05 when compared with BaP1 and BlatH1. In (A,B), the signal of BlatPII was not significantly different when compared to the controls without enzyme (p > 0.05); (C) Gelatin zymography: 2.5 µg of BlatPII (Lane 2) and 2.5 µg of BlatH1 (Lane 3) were separated on SDS-PAGE under non-reducing conditions. Lane 1 corresponds to molecular mass standards. The dark band observed in Lane 2 corresponds to BlatPII, whose migration in SDS-PAGE-gelatin gels is delayed as compared to SDS-PAGE gels without gelatin.