Table 2.
Scope of palladium-catalyzed C–H arylation of triazoles.
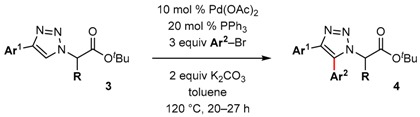
| Entry | Ar1 | Ar2 | R | Yield |
|---|---|---|---|---|
| 1 | 4-pyridyl | phenyl | H | 89% |
| 2 | 4-pyridyl | 4-MeO-phenyl | H | 85% |
| 3 | 4-pyridyl | 4-EtO2C-phenyl | H | 92% |
| 4 | 4-pyridyl | 4-F3C-phenyl | H | 83% |
| 5 | 4-pyridyl | 4-NC-phenyl | H | 79% |
| 6 | 4-pyridyl | 4-F-phenyl | H | 51% |
| 7 | 4-pyridyl | 3-Me-phenyl | H | 86% |
| 8 | 4-pyridyl | 3-OHC-phenyl | H | 32% |
| 9 | 4-pyridyl | 2-MeO-phenyl | H | 82% |
| 10 | 4-pyridyl | 2-Me-phenyl | H | 49% |
| 11 | 4-pyridyl | 1-naphthyl | H | 78% |
| 12 | 4-pyridyl | phenyl | H | 80% |
| 13 | 4-pyridyl | phenyl | H | 84% |
| 14 | phenyl | phenyl | H | 80% |
| 15 | 4-MeO-phenyll | phenyl | H | 64% |
| 16 | 2-F3C-phenyl | phenyl | H | 50% |
| 17 | 4-pyridyl | phenyl | Me | 20% a |
| 18 | 4-pyridyl | phenyl | Et | 8% a |
a Microwave heating, 140 °C, 15 min.
