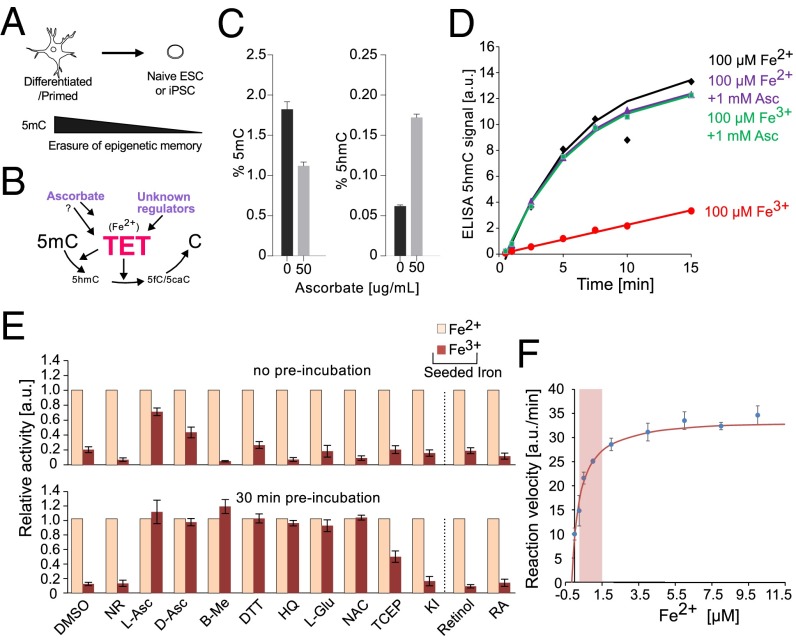
Fig. 1.
TET protein catalytic activity in vitro is rescued by the addition of Fe2+, ascorbate, or other antioxidants. (A) Major loss of DNA methylation, globally and at gene promoters, is associated with naïve pluripotent cells. (B) Demethylation during reprogramming is affected by the activity of the Fe2+-dependent TET hydroxylases, which create 5hmC and other oxidized derivatives (5fC and 5caC). However, what regulates TET levels is largely unknown, and the mechanism by which factors such as ascorbate affect TET-mediated oxidation are unclear. (C) Global levels of 5mC and 5hmC (%) in nESCs following 72 h of supplementation with 50 μg/mL ascorbate (± SD; n = 3). (D) Kinetics of TET1-CD–mediated 5hmC production when supplemented with 100 μM iron (Fe2+ or Fe3+) and 1 mM ascorbate (corresponds to 172.12 μg/mL). (E, Upper) Relative activity of TET1-CD when supplemented with 100 μM iron (Fe2+ or Fe3+) and various antioxidants. (E, Lower) The same experiment repeated, with the antioxidant and iron mix preincubated for 30 min before the addition of TET1-CD (± SD; n = 4). NR represents reaction conditions without added reducing agents. (F) Relative activity of TET1-CD at various Fe2+ concentrations encompassing those seen in cellular contexts (0.2–1.5 μM, indicated by a red box). The mean apparent dissociation constant (Kd) for this reaction was determined to be 0.41 ± 0.05 µM (n = 2).