Significance
Orthopedic surgery sometimes causes persistent pain and, especially in the elderly, delirium and cognitive dysfunction. Using an established mouse bone-fracture model associating with memory deficits, we assessed pain behavior and expression of several molecules in sensory neurons, spinal cord, and brain. An increase in cold sensitivity and up-regulation of several injury markers, including activating transcription factor 3, the neuropeptide galanin, and growth factor brain-derived neurotrophic factor (BDNF), were observed in sensory ganglia. In the hippocampus, BDNF protein levels were increased in mossy fibers. In contrast, the Bdnf transcript was not increased in the parent granule cell bodies, and c-Fos levels were decreased, as was neurogenesis. Thus, impaired hippocampal BDNF signaling may contribute to mental deficits observed after surgery.
Keywords: postoperative pain, nerve injury, memory, delirium, neurogenesis
Abstract
Pain is a critical component hindering recovery and regaining of function after surgery, particularly in the elderly. Understanding the role of pain signaling after surgery may lead to novel interventions for common complications such as delirium and postoperative cognitive dysfunction. Using a model of tibial fracture with intramedullary pinning in male mice, associated with cognitive deficits, we characterized the effects on the primary somatosensory system. Here we show that tibial fracture with pinning triggers cold allodynia and up-regulates nerve injury and inflammatory markers in dorsal root ganglia (DRGs) and spinal cord up to 2 wk after intervention. At 72 h after surgery, there is an increase in activating transcription factor 3 (ATF3), the neuropeptides galanin and neuropeptide Y (NPY), brain-derived neurotrophic factor (BDNF), as well as neuroinflammatory markers including ionized calcium-binding adaptor molecule 1 (Iba1), glial fibrillary acidic protein (GFAP), and the fractalkine receptor CX3CR1 in DRGs. Using an established model of complete transection of the sciatic nerve for comparison, we observed similar but more pronounced changes in these markers. However, protein levels of BDNF remained elevated for a longer period after fracture. In the hippocampus, BDNF protein levels were increased, yet there were no changes in Bdnf mRNA in the parent granule cell bodies. Further, c-Fos was down-regulated in the hippocampus, together with a reduction in neurogenesis in the subgranular zone. Taken together, our results suggest that attenuated BDNF release and signaling in the dentate gyrus may account for cognitive and mental deficits sometimes observed after surgery.
Fractures represent a common clinical problem and remain a leading cause of morbidity, in particular among the rapidly growing elderly population requiring surgery (1). Although generally well-tolerated, orthopedic surgery is traumatic and may lead to poor functional outcomes, including mortality and prolonged recovery and rehabilitation, especially in frail aged patients with concurrent medical problems (2, 3).
Postoperative pain, not rarely developing into chronic pain, is a common disabling complication after surgery, which dramatically reduces quality of life and represents a major burden for both patients and society. Poorly managed postoperative pain is a leading cause of acute confusional state (delirium) (4), which affects more than 50% of patients undergoing hip-fracture repair (5). Notably, 10 to 26% of surgical patients retain subtle but persistent learning and memory deficits, referred to as postoperative cognitive dysfunction (POCD) (6), which results in further morbidity and greater risks for permanent dementia (7). Cognitive impairment is a critical component of the pain experience (8), and patients with chronic pain also suffer from deficits in learning and memory (9). Recent studies have independently shown hippocampal abnormalities in animal models of neuropathic pain and reduced hippocampal volume in elderly patients with chronic pain (10–12). Moreover, changes in regional brain volume, including hippocampal and cortical atrophy, have also been related to POCD (13), although the etiology of this phenomenon remains poorly understood.
Tibial fracture is an established experimental rodent model to study clinically relevant orthopedic procedures (14) and a variety of clinical pathologies ranging from postoperative pain, complex regional pain syndrome, and POCD to bone cancer (15–19).
After fracture, nociceptive sensations have been associated with excessive substance P signaling and exaggerated regional inflammatory responses leading to the increased release of systemic proinflammatory cytokines such as tumor necrosis factor alpha and interleukin 1 beta (18, 20, 21). Similar changes in proinflammatory cytokines and activation of nuclear factor κB signaling in macrophages have been associated with changes in blood–brain barrier permeability, hippocampal neuroinflammation, and subsequent cognitive impairment in mice after tibial fracture (22–24). However, whether there are neuronal substrates for pain and cognitive impairment after tibial fracture remains largely unknown.
Here we therefore characterized the effects of tibial fracture with intramedullary pinning on the primary somatosensory system, including dorsal root ganglia and spinal cord, by combining pain behavioral tests with histochemical analyses of an array of markers for nerve injury, including neuropeptides, brain-derived neurotrophic factor (BDNF), and c-Fos for a period of 2 wk postoperatively. For comparison, the same markers were assessed after a complete transection (axotomy) of the sciatic nerve. We also analyzed these markers in the brain with special focus on the hippocampal formation to explore a possible relationship between peripheral surgery, pain, and cognitive deficits. Taken together, our results show major and distinct changes of pain-related markers at the spinal level and in the hippocampal formation, providing a new view of the complex and widespread effects induced by tibial fracture with pinning.
Results
Tibial Fracture with Pinning Induces Cold Allodynia.
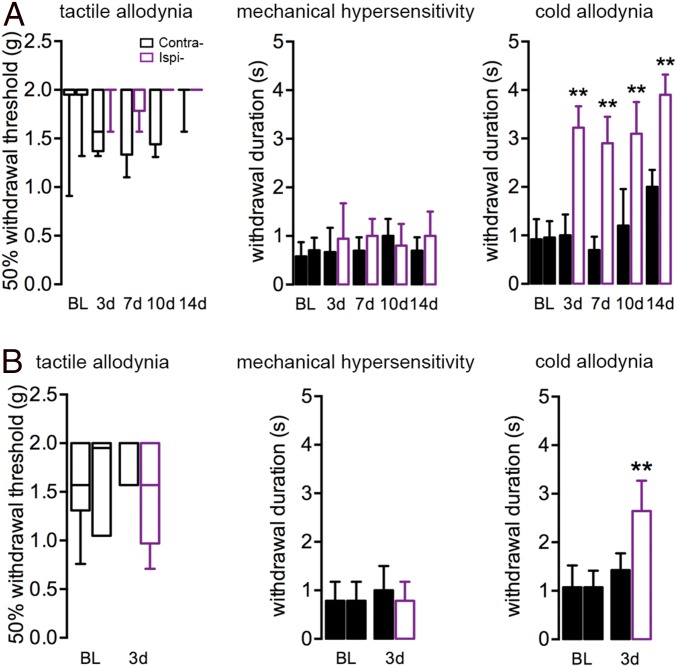
Neuropathic pain resulting from injury to peripheral nerves is a common clinical consequence of surgery (15). In animal models, neuropathic pain is characterized by changes in tactile, cold allodynia, and mechanical hypersensitivity, as induced for example by partial nerve injury to the sciatic nerve (spared nerve injury) (25). To test whether tibial fracture with pinning triggers pain behavior, we performed von Frey hair (nonnoxious tactile stimulus), pinprick (noxious mechanical stimulus), and acetone (cold stimulus)-induced behavioral sensitization tests. Mice showed robust cold allodynia 3 d after orthopedic surgery, which lasted for at least 2 wk (Fig. 1A). However, neither mechanical hypersensitivity nor tactile allodynia was observed until the end of 2 wk (Fig. 1A). In fact, no response to the cutoff value of von Frey hair stimulus was observed ipsilaterally. Furthermore, the sham group (no bone pinning or fracture) also developed modest cold allodynia but neither tactile allodynia nor mechanical hypersensitivity (Fig. 1B).
Fig. 1.
Cold allodynia triggered by unilateral tibial fracture. (A) Mice with fracture do not develop tactile allodynia or mechanical hypersensitivity after surgery but have exaggerated responses to cold stimulus from day 3 (3d), lasting for at least the 14 d (14d) that were recorded in this study. (B) Mice processed for sham surgery also develop mild cold allodynia. BL, baseline; Contra, contralateral; Ipsi-, ipsilateral. Data for tactile allodynia from von Frey filament tests (nonparametric data) are represented as box and whisker plots (analyzed with unpaired Mann–Whitney test), whereas withdrawal durations for pinprick and acetone stimuli are represented as mean ± SD. Experimental group, n = 5–9; sham group, n = 6. **P < 0.01; analyzed with unpaired t test.
Nerve Injury in Tibial Fracture with Pinning Compared with Axotomy.
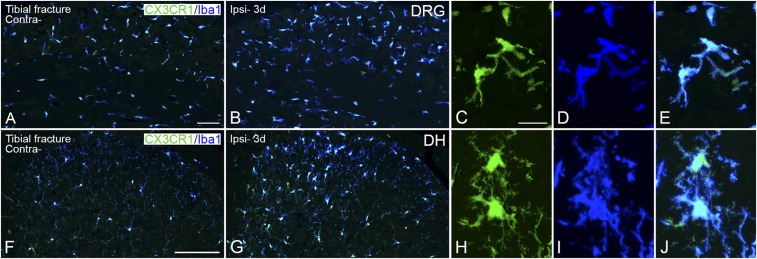
To estimate the severity of nerve damage, we compared the expression of several markers in neurons, such as activating transcription factor 3 (ATF3), the neuropeptides galanin and neuropeptide Y (NPY), and the growth factor BDNF as well as the three glial markers glial fibrillary acidic protein (GFAP) (astrocytes), ionized calcium-binding adaptor molecule 1 (Iba1) (macrophages/microglia), and the fractalkine receptor CX3CR1 (microglia), in dorsal root ganglia (DRGs) and spinal cord with the effects of axotomy, a classical model of “severe” peripheral nerve injury.
Neuronal Markers.
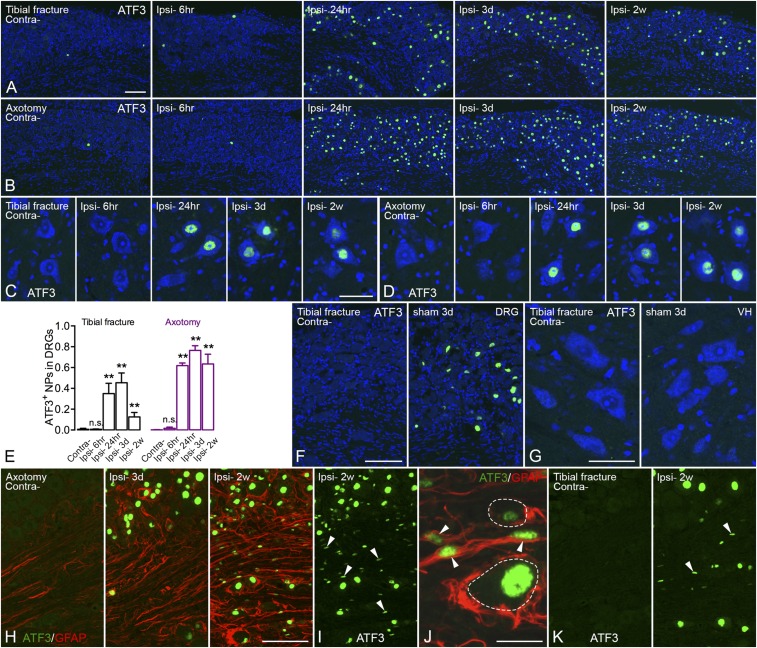
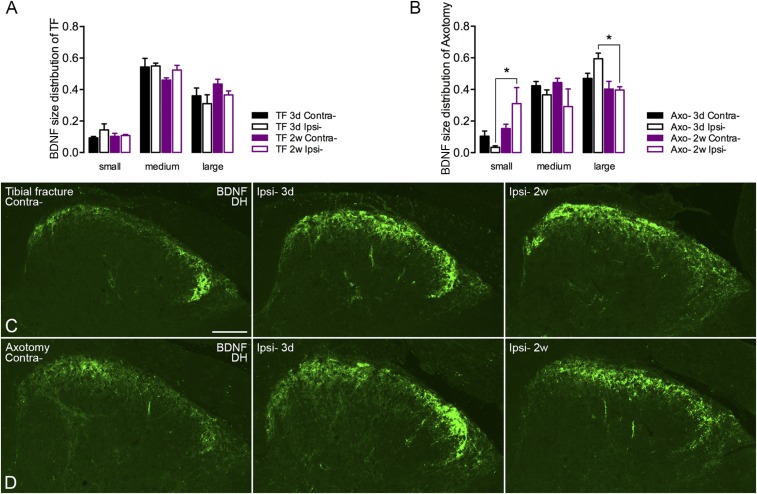
ATF3 was usually not detected, or only occasionally, in control DRG neurons (Fig. 2 A and B). ATF3 was up-regulated in 35 ± 10% of neuron profiles (NPs) as early as 24 h after tibial fracture with pinning and up to 45 ± 9% of NPs after 3 d, but dropped to 13 ± 4% after 2 wk (Fig. 2 A and E). In axotomized DRGs, 62 ± 3% of NPs were ATF3-immunoreactive (IR) after 24 h and up to 76 ± 5% after 3 d, and this level was essentially maintained at 2 wk (63 ± 9%) (Fig. 2 B and E). Motor neurons in the spinal ventral horn started to express ATF3 24 h after injury in both orthopedic and axotomy models, remaining elevated at least for 2 wk, although less so after fracture (Fig. 2 C and D). In the tibial fracture sham group, a small population of ATF3-IR neurons was also observed in DRGs (9 ± 4%) (Fig. 2F) but not in motor neurons (Fig. 2G). Furthermore, ATF3-like immunoreactivity (LI) was observed in a number of small nuclei (rod-shaped) tightly surrounded by GFAP-LI, presumably in Schwann cells, 2 wk after axotomy (Fig. 2 H–J). This was also observed after tibial fracture with pinning, but in fewer cells (Fig. 2K).
Fig. 2.
Activation of ATF3 in DRGs after unilateral tibial fracture. (A) Expression of ATF3 (green) is induced in DRGs 24 h after tibial fracture and declines after 2 wk, whereas contralateral DRGs do not (or very rarely) express ATF3. (B) Axotomy induces ATF3 expression in a larger population of DRG cells, essentially neurons, after 24 h, and is maintained for at least 2 wk. (C and D) Motor neurons in the spinal ventral horn start to express ATF3 at 24 h, lasting for at least 2 wk in both tibial fracture and axotomy models. (E) Quantification of the number of ATF3-IR neuronal nuclei after tibial fracture and axotomy. (F and G) In the sham group, ATF3 is induced in a small population of DRG neurons but not in spinal motor neurons (3 d). (H) Overview of double labeling with ATF3 (green) and GFAP (red) in DRGs 3 d and 2 wk after axotomy. (I and J) Overview of weakly activated ATF3 (green) in Schwann/satellite cells (indicated by arrowheads) after 2 wk, and high magnification of double labeling of ATF3 (green) and GFAP (red), where arrowheads indicate coexpression. (K) Overview of ATF3 (green) in DRGs after 2 wk of tibial fracture (arrowheads indicate a few rod-shape nuclei, as in I and J). Counterstaining with propidium iodide (blue) (A–D, F, and G). n = 37; n = 4, 5, 4, and 5 for axotomy groups (different time points from shortest to longest, respectively); n = 3, 6, 6, and 4 for tibial fracture groups. Data are represented as mean ± SD. **P < 0.01; analyzed with unpaired t test. n.s., no significant difference. [Scale bars, 100 μm (A and B), 50 μm (C and D), 100 μm (F), 50 μm (G), 100 μm (H, I, and K), and 20 μm (J).]
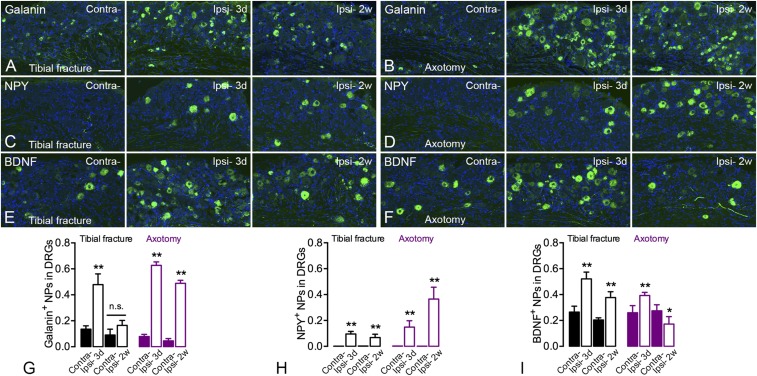
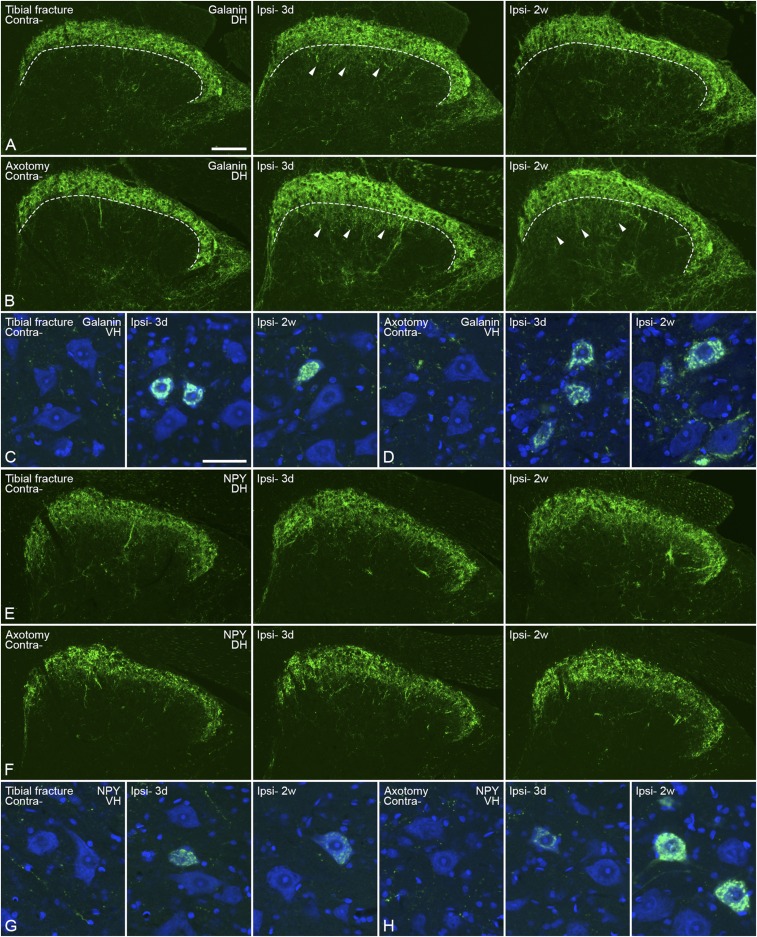
A basal expression level of galanin-LI was found in 5 to 10% of DRG neurons. Three days after orthopedic surgery, the proportion of galanin-IR NPs was increased ipsilaterally from 14 ± 3% to 48 ± 8%, but dropped to 16 ± 4% after 2 wk (Fig. 3 A and G). After axotomy, the levels of galanin-LI were increased after 3 d and remained up-regulated for 2 wk, although slightly reduced (3 d 63 ± 3% vs. 2 wk 49 ± 2%) (Fig. 3 B and G). In the spinal dorsal horn, there was an increase of galanin-LI, also in motor neurons (Fig. S1 A–D). The increase in the dorsal horn was evident in the deeper layers, and was stronger after axotomy than tibial fracture as measured both at 3 d and 2 wk (Fig. S1 A and B).
Fig. 3.
Increased expression of galanin, NPY, and BDNF in DRG neurons after unilateral tibial fracture. (A–D) Both galanin- (A and B) and NPY- (C and D) LIs show increased expression in DRG neurons, more so after axotomy than fracture. The quantification of neuron profiles is shown in G and H, respectively. (E and F) BDNF-LI is increased in DRG neurons after fracture but only weakly increased after 3 d of axotomy and not at all after 2 wk, as shown by the quantification in I. Neuronal markers are in green; counterstaining with propidium iodide is in blue. n = 19; n = 4 and 5 for axotomy groups; n = 4 and 6 for tibial fracture groups. Data are represented as mean ± SD. *P < 0.05, **P < 0.01; analyzed with unpaired t test. (Scale bars, 100 μm.)
Fig. S1.
Expression of galanin- and NPY-LI in the spinal cord 3 d (3d) or 2 wk (2w) after unilateral injury. (A and B) Galanin-LI (green) in the spinal dorsal horn after tibial fracture and axotomy, respectively. Dashed lines indicate the border between the outer and inner layer of lamina II. Arrowheads indicate increased galanin-LI in deep layers of the medial dorsal horn. (C and D) Increased galanin-LI in motor neurons after tibial fracture and axotomy, respectively. (E and F) NPY-LI (green) in the spinal dorsal horn after tibial fracture and axotomy, respectively. (G and H) Increased NPY-LI in motor neurons after tibial fracture and axotomy, respectively. Counterstaining with propidium iodide (blue) (C, D, G, and H). Contra, contralateral; DH, dorsal horn; Ipsi-, ipsilateral; VH, ventral horn. [Scale bars, 100 μm (A, B, E, and F) and 50 μm (C, D, G, and H).]
NPY is challenging to detect in DRG neurons with immunohistochemistry (IHC). The expression of NPY was slightly increased in DRGs 3 d after tibial fracture with pinning (10 ± 2%) and was maintained for at least 2 wk (7 ± 3%) (Fig. 3 C and H). The increase of NPY-IR NPs after axotomy was also long-lasting but much stronger after 2 wk (3 d 15 ± 5% vs. 2 wk 37 ± 9%) (Fig. 3 D and H). In the spinal cord, a clear increase in NPY-LI could be seen in motor neurons, both after orthopedic surgery and axotomy, but no evident changes were observed in the dorsal horn (Fig. S1 E–H).
For BDNF, the expression was increased in DRG neurons 3 d after tibial fracture with pinning and maintained for 2 wk, even if slightly reduced (3 d contralateral 26 ± 5% vs. ipsilateral 52 ± 5% and 2 wk contralateral 20 ± 2% vs. ipsilateral 36 ± 5%) (Fig. 3 E and I). Also after axotomy, BDNF was up-regulated after 3 d (contralateral 26 ± 6% vs. ipsilateral 39 ± 2%) (Fig. 3 F and I), but this up-regulation did not persist after 2 wk, and in fact was reduced below controls (contralateral 28 ± 5% vs. ipsilateral 17 ± 6%) (Fig. 3 F and I), contrasting the effect of orthopedic surgery (Fig. 3 E and I). Morphometric analysis of BDNF-IR neurons showed a slight effect in the shift of small- to large-sized neurons after axotomy (Fig. S2 A and B), similar to a previous study in rat DRGs (26), but this shift was not observed following tibial fracture. In the spinal dorsal horn, BDNF-LI was increased in both models, although stronger after 3 d than 2 wk (Fig. S2 C and D).
Fig. S2.
Size distribution of BDNF-IR DRG neurons and expression of BDNF in the spinal dorsal horn 3 d or 2 wk after unilateral injury. (A) Size distribution of BDNF-IR DRG neurons after tibial fracture (TF). (B) Size distribution of BDNF-IR DRG neurons after axotomy. (C and D) BDNF-LI is increased both after tibial fracture (C) and axotomy (D). Data are represented as mean ± SEM. *P < 0.05; one-way ANOVA followed by Tukey’s multiple comparison test was applied for different categories of DRG neurons. (Scale bar, 100 μm.)
Glial Markers.
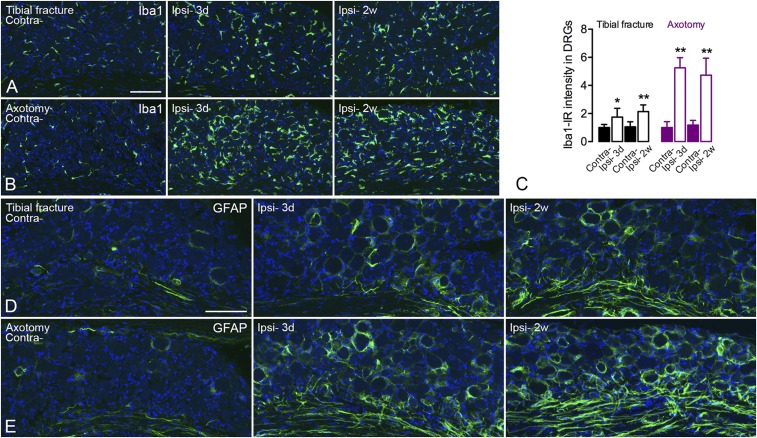
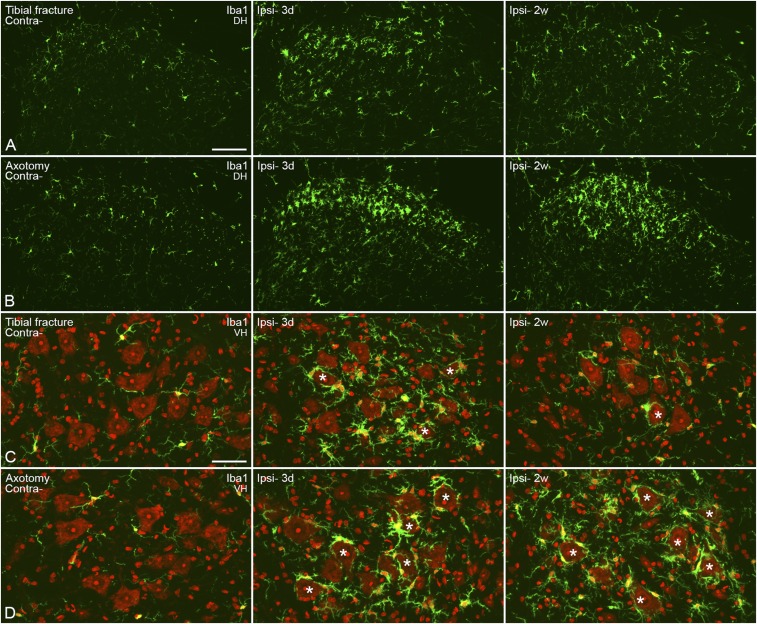
After tibial fracture with pinning, Iba1 was increased (intensity of Iba1-LI) in DRG microglia/macrophages 3 d after surgery (contralateral 1.00 ± 0.22-fold vs. ipsilateral 1.75 ± 0.63-fold) with sustained activity even after 2 wk (contralateral 1.06 ± 0.36-fold vs. ipsilateral 2.14 ± 0.48-fold) (Fig. S3 A and C). However, the activation was more evident after axotomy (3 d contralateral 1.00 ± 0.43-fold vs. ipsilateral 5.26 ± 0.71-fold and 2 wk contralateral 1.18 ± 0.33-fold vs. ipsilateral 4.73 ± 1.21-fold) (Fig. S3 B and C). In the dorsal horn, microglial activation was transient after tibial fracture with pinning but long-lasting after axotomy (Fig. S4 A and B). A similar pattern was also observed in the ventral horn, but exclusively around motor neurons (Fig. S4 C and D).
Fig. S3.
Increased expression of Iba1-LI in microglia and of GFAP-LI in satellite glia/Schwann cells in DRGs after unilateral injury. (A and B) Confocal pictures show activated Iba1-IR (green) microglia 3 d and 2 wk after tibial fracture and axotomy, respectively. (C) Quantification of the intensity of Iba1-LI. (D and E) Confocal pictures show activated GFAP-IR (green) satellite glia and Schwann cells 3 d and 2 wk after tibial fracture and axotomy, respectively. Counterstaining with propidium iodide (blue) (A, B, D, and E). n = 19; n = 4 and 5 for axotomy groups; n = 4 and 6 for tibial fracture groups. Data are represented as mean ± SD. *P < 0.05, **P < 0.01; analyzed with unpaired t test. [Scale bars, 100 μm (A and B) and 50 μm (C and D).]
Fig. S4.
Activation of microglia in the spinal cord 3 d and 2 wk after unilateral injury. (A and B) Confocal micrographs show activation of microglia in the dorsal horn after fracture, with regard to both morphology and numbers (A) and more so after axotomy (B). (C and D) Projection micrographs show activated cells in the ventral horn, especially in the area surrounding motor neurons. After fracture, an increase is only seen after 3 d; but the activation of microglia is maintained even after 2 wk of axotomy. Counterstaining with propidium iodide (red) (C and D). Projection micrographs are produced from z-stack scanning of six series pictures with an interval of 2 μm. Asterisks indicate motor neurons surrounded by activated microglia. [Scale bars, 100 μm (A and B) and 50 μm (C and D).]
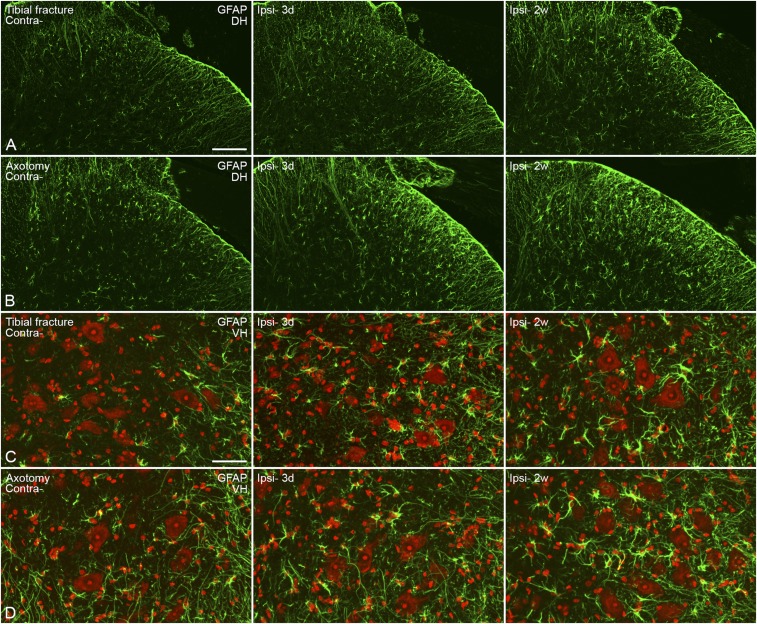
The up-regulation of GFAP-LI in satellite glia cells of DRGs was observed 3 d and 2 wk after orthopedic surgery (Fig. S3D), again more profound after axotomy than tibial fracture with pinning (Fig. S3 D and E). Up-regulation of GFAP-LI was also observed in Schwann cells both after tibial fracture with pinning and axotomy (Fig. S3 D and E). A modest astrocyte activation was seen in the dorsal horn and around motor neurons after tibial fracture with pinning (Fig. S5 A and C), with a somewhat more robust activation after axotomy (Fig. S5 B and D). The latter response was stronger at 2 wk than 3 d (Fig. S5 B and D).
Fig. S5.
Activation of astrocytes in the spinal cord 3 d and 2 wk after unilateral tibial fracture or axotomy. (A and B) Confocal micrographs show modest activation of astrocytes in the dorsal horn, with regard to both morphology and numbers (A) and more so after axotomy (B). (C and D) Projection micrographs show activated cells in the ventral horn, especially in the area surrounding motor neurons (C), again stronger after axotomy (D). Counterstaining with propidium iodide (red) (C and D). Projection pictures are produced from z-stack scanning of six series pictures with an interval of 2 μm. [Scale bars, 100 μm (A and B) and 50 μm (C and D).]
The fractalkine receptor (CX3CR1), mediating inflammatory and neuropathic pain (27, 28), was up-regulated in DRGs (Fig. S6 A–E) and spinal dorsal horn (Fig. S6 F–J) 3 d after tibial fracture with pinning, coinciding with the expression of Iba1 (Figs. S3A and S4 A and B).
Fig. S6.
Increased expression of CX3CR1-LI in microglia in DRGs and spinal dorsal horn 3 d after unilateral tibial fracture. Confocal micrographs show double staining for Iba1-LI (blue) and CX3CR1 (green) in activated microglia in DRGs (A–E) and dorsal horn (F–J). High-magnification, projection micrographs show colocalization of CX3CR1/Iba1 in microglia from the ipsilateral ganglion (C–E) and in the ipsilateral dorsal horn (medial part) (H–J). Projection micrographs are produced from z-stack scanning of 13/14 series micrographs with an interval of 1 μm. [Scale bars, 200 μm (A and B), 500 μm (F and G), and 20 μm (C–E and H–J).]
For a direct and convenient comparison between orthopedic surgery and axotomy, detailed quantification of the neuronal and glial markers in DRGs is summarized in Table S1 (3 d and 2 wk).
Table S1.
Summary for the quantification of neuronal and glial markers in DRGs
| Marker | Three days | Two weeks | ||||||
| Tibial fracture | Axotomy | Tibial fracture | Axotomy | |||||
| Contra | Ipsi- | Contra | Ipsi- | Contra | Ipsi- | Contra | Ipsi- | |
| ATF3 | 0 | 45 ± 9 | 0 | 76 ± 5 | 0 | 13 ± 4 | 0 | 63 ± 9 |
| Galanin | 14 ± 3 | 48 ± 8 | 7 ± 3 | 63 ± 3 | 11 ± 3 | 16 ± 4 | 5 ± 2 | 49 ± 2 |
| NPY | 0 | 10 ± 2 | 0 | 15 ± 5 | 0 | 7 ± 3 | 0 | 37 ± 9 |
| BDNF | 27 ± 5 | 52 ± 5 | 26 ± 6 | 39 ± 2 | 20 ± 2 | 36 ± 5 | 28 ± 5 | 17 ± 6 |
| Iba1 | + | ++ | + | ++++ | + | ++ | + | ++++ |
| GFAP | + | ++ | + | +++ | + | ++ | + | ++++ |
Numbers stand for the neuronal percentage (%). Bolding indicates the dropping of BDNF expression. +, expression abundance.
c-Fos in the Spinal Cord and Hippocampal Formation.
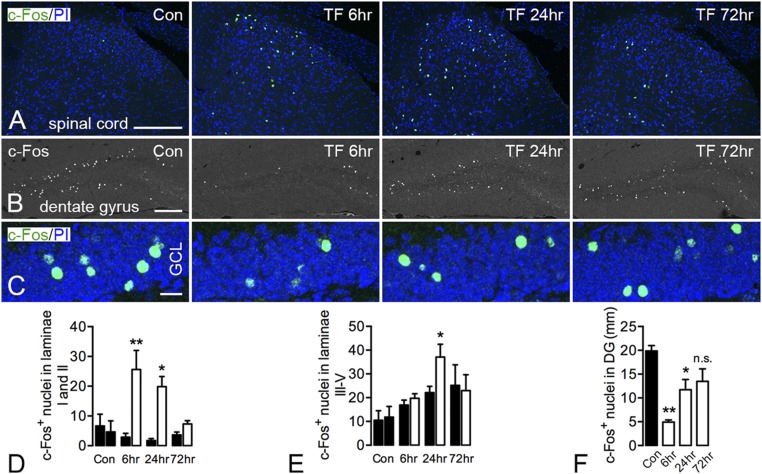
c-Fos, an immediate-early transcription factor (29), was early on shown to be “induced” in the dorsal horn following sensory stimulation (30). After tibial fracture with pinning, c-Fos activation occurred unilaterally in the superficial layer of the spinal dorsal horn from 6 to 24 h, declining to basal levels after 3 d (Fig. 4 A and D). However, the activation of c-Fos in deep layers of the spinal cord (laminae III–V) gradually increased on both contra- and ipsilateral sides. However, the increase of c-Fos–positive nuclei in the ipsilateral side was only significant at 24 h after fracture compared with the contralateral side (Fig. 4 A and E).
Fig. 4.
Modulation of c-Fos expression in the spinal cord and hippocampal formation after unilateral tibial fracture. (A) Robust activation of c-Fos in the superficial layers (laminae I and II) of the dorsal horn peaking at 6 h after fracture and declining to basal levels after 72 h. The activation of c-Fos in deep layers of the spinal cord appears increased bilaterally, but significance is reached only at 24 h compared with the contralateral side. (B and C) c-Fos expression in granule cells of the dentate gyrus is decreased transiently (6, 24 h) after tibial fracture, returning to basal levels after 72 h, as substantiated by quantification. Only the number of c-Fos–positive nuclei was counted, without considering intensity. Projection pictures in C are produced from 12- to 14-μm z-stack scanning with an interval of 2 μm. Con, control; GCL, granule cell layer; PI, propidium iodide; TF, tibial fracture. n = 14; n = 3 for the control group; n = 4 for both 6- and 24-h injury; and n = 3 for 72-h injury. Data are represented as mean ± SD. *P < 0.05, **P < 0.01; unpaired t test was applied for c-Fos in the spinal cord, and one-way ANOVA followed by Dunnett’s multiple comparison test was applied for c-Fos in the hippocampus with tibial fracture. [Scale bars, 200 μm (A and B) and 20 μm (C).]
c-Fos is dramatically up-regulated in the hippocampus in response to seizure activity (31). In contrast, in the present model, c-Fos was bilaterally decreased in hippocampal cell layers as early as 2 h after surgery. In the dentate granule cellular layer, c-Fos was decreased 6 h after tibial fracture with pinning, with a gradual return to basal levels after 3 d (Fig. 4 B, C, and F). This also contrasts with the acute activation in the spinal cord in the present study.
BDNF and Doublecortin in the Hippocampal Formation.
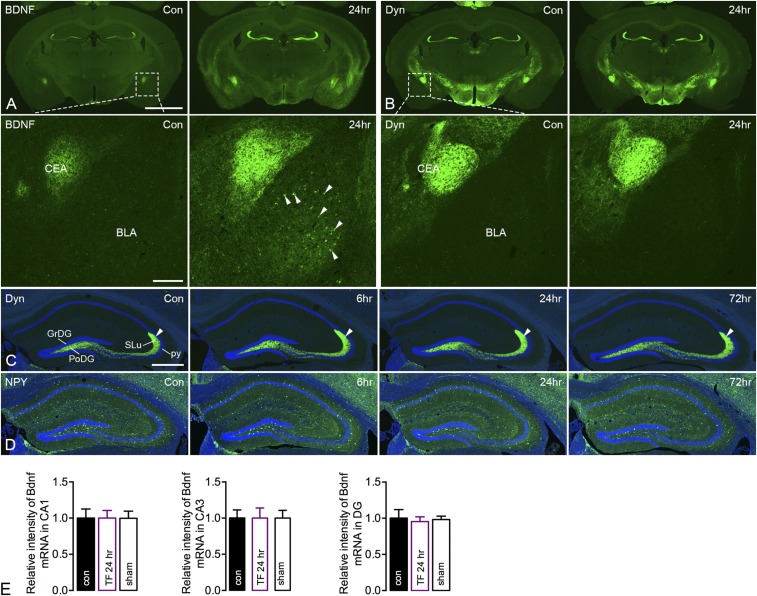
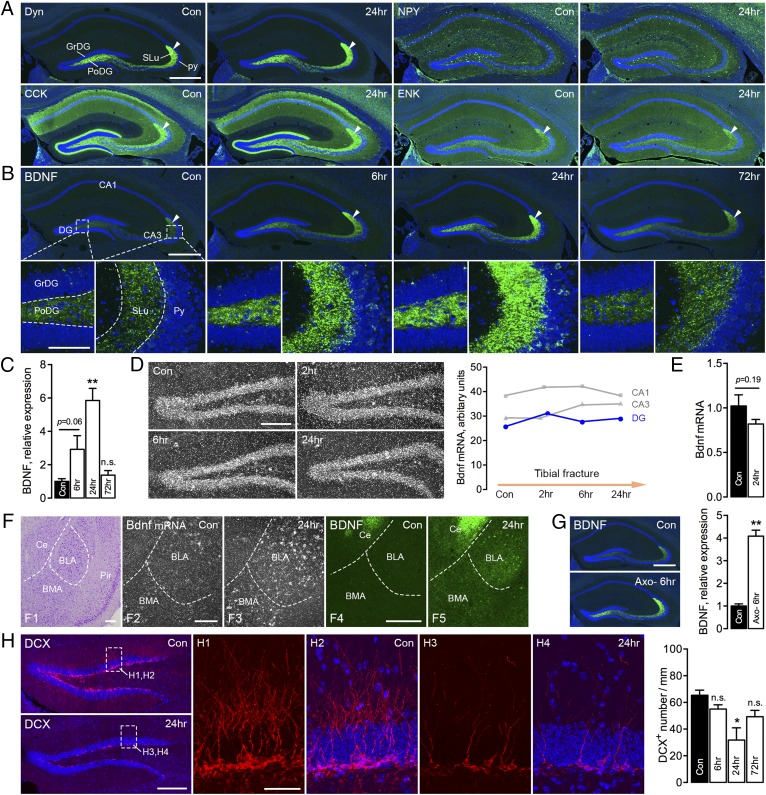
Our description of the distribution of BDNF protein (IHC) (32) (same Amgen antibody as used in the present study) and transcript (in situ hybridization; ISH) (33) confirms and is in agreement with early histochemical analyses. After orthopedic surgery, BDNF-LI was distinctly, bilaterally, and transiently increased in the polymorph layer and stratum lucidum of CA3 (mossy fibers), as well as in many other brain regions, such as the subnuclei of the amygdaloid complex (Fig. S7A). A similar pattern was seen after incubation with a second primary antibody (mAb#9), which has been thoroughly characterized and shown to recognize both mature BDNF and proBDNF (34). However, classical neuropeptides, such as dynorphin and NPY, both known to be strongly up-regulated by seizure activity (35), were not modulated by tibial fracture with pinning (Fig. 5A and Fig. S7 B–D). This was true also for two further neuropeptides, cholecystokinin and enkephalin (Fig. 5A).
Fig. S7.
Effects of unilateral tibial fracture on BDNF-, dynorphin (Dyn)-, and NPY-LIs in coronal brain sections. (A) Increase of BDNF-LI in the hippocampal dentate gyrus, as clearly shown in the overview 24 h after injury, as well as at high magnification (boxed area in A) in the central (CEA) or basolateral (BLA) amygdaloid nuclei. Note that the increases are bilateral. The arrowheads indicate BDNF-IR neurons. (B–D) Dyn-LI 24 h after injury. No certain effects can be observed in any region, not even at high magnification in CEA or BLA (boxed area in B). This is true for various time intervals, where the mossy fibers are indicated by arrowheads (C), and also for NPY (D). (E) Bdnf mRNA levels in hippocampal layers on X-ray film from ISH. Data are represented as mean ± SD. Counterstaining with propidium iodide (blue) (C and D). Con, control. [Scale bars, 500 μm (A and B), 200 μm (A and B, Insets), and 200 μm (C and D).]
Fig. 5.
BDNF and neuropeptide expression in the brain after unilateral tibial fracture. (A) Overview micrographs of dynorphin (Dyn; green), NPY, cholecystokinin (CCK), and enkephalin (ENK) in the hippocampus, with emphasis on the polymorph layer of the dentate gyrus (PoDG) and stratum lucidum (SLu) of CA3 from control and mice with tibial fracture (24 h). Arrowheads indicate mossy fibers in SLu. (B) Overview and high-magnification micrographs show increased levels of BDNF-LI (green) in the PoDG and SLu of CA3 from the hippocampal formation at different time points after tibial fracture. Boxed rectangles show PoDG and SLu in CA3. High-magnification micrographs are produced from z-stack scanning with an interval of 2 μm (projected from nine series pictures). GrDG, granular layer of the DG; Py, pyramidal layer. (C) Quantification of BDNF-LI in the hippocampus after tibial fracture. (D) Dark-field micrographs of a [35S]UTP-labeled Bdnf antisense probe in the dentate gyrus after tibial fracture. Quantification of silver particles for Bdnf mRNA is represented by a plot graph. (E) Quantification of Bdnf transcript levels in the hippocampal formation (RT-qPCR) shows the fold changes of Bdnf mRNA levels after tibial fracture. (F) Cresyl violet staining micrograph shows central amygdaloid (Ce), basolateral (BLA), and basomedial (BMA) nuclei and piriform cortex (Pir). Dark-field micrographs show an increased signal for a [35S]UTP-labeled Bdnf antisense probe, mainly in the BLA, 24 h after tibial fracture (F2 and F3). Confocal pictures show increased BDNF-IR neurons in the Ce and BLA (F4 and F5). (G) Overview micrographs of BDNF-LI in the dentate gyrus 6 h after axotomy, and quantification of BDNF-LI. (H) Overviews show DCX-LI (red) in the dentate gyrus 24 h after tibial fracture. Boxes show projection micrographs from control and 24 h after tibial fracture. Quantification of DCX+ neurons shows the number of cell bodies per mm from one airy unit pinhole of 10× objective. The high-power micrographs are projected from z-stack scanning of 12-μm-thick tissue with an interval of 2.0 μm. Counterstaining with propidium iodide (blue) (A, B, and H). n = 38; n = 4 and 4 for control and axotomy groups; n = 3–4 (n = 14) for control and tibial fracture groups with different time points for IHC; n = 4 and 4 for control and tibial fracture 24 h for qPCR; and n = 2 (n = 8) for control and tibial fracture groups with different time points for ISH. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01; one-way ANOVA followed by Dunnett’s multiple comparison test was applied for the intensity of both BDNF and DCX in the hippocampus with tibial fracture. Bdnf qPCR data and the intensity of BDNF in the hippocampus after axotomy were analyzed with unpaired t test. [Scale bars, 500 μm (A and B), 100 μm (B, Insets), 200 μm (D), 200 μm (F), 500 μm (G), 200 μm (H), and 50 μm (H, Insets).]
An apparent increase in BDNF-LI was already seen after 6 h (although not significant, P = 0.06), peaking at 24 h, and then almost returning to control levels after 3 d (fold changes: control, 1.00 ± 0.16; 6 h, 2.92 ± 0.82; 24 h, 5.85 ± 0.73; 72 h, 1.38 ± 0.24) (Fig. 5 B and C). However, Bdnf mRNA levels in the dorsal hippocampal formation were not changed when analyzed either by ISH (pyramidal cells, granule cells) at any time point (2, 6, 24 h) (Fig. 5D) or by RT-quantitative (q)PCR (hippocampal samples) at the peak of BDNF protein content (fold changes: control, 1.02 ± 0.13; 24 h, 0.82 ± 0.05) (Fig. 5E). This result was confirmed in a second ISH experiment measuring Bdnf mRNA levels in all main hippocampal layers (CA1, CA3, granule cells) on X-ray film (Fig. S7E).
An increase in BDNF-LI was also observed in the central amygdala (mainly nerve endings), as was an increased number of BDNF-IR neurons in the basolateral nucleus, an increase also seen in many other brain regions (Fig. 5F and Fig. S7A). However, in contrast to the granule cells/mossy fibers in the dentate gyrus, both Bdnf mRNA and protein levels were increased in the basolateral nucleus (Fig. 5F, F2 and F3 for Bdnf mRNA; Fig. 5F, F4 and F5 show BDNF IHC for comparison). An acute increase of BDNF-LI in mossy fibers was also found after axotomy, peaking at 6 h (fold changes: control, 1.00 ± 0.10; 6 h, 4.09 ± 0.27) (Fig. 5G), but returned to basal levels already after 24 h. Thus, changes in BDNF protein were in this case more prolonged after tibial fracture than axotomy. Furthermore, the number of doublecortin (DCX)-stained cells and their dendritic processes (IHC) was significantly reduced in the dentate gyrus 24 h after tibial fracture with pinning, returning to control levels after 3 d (Fig. 5H).
Discussion
For many decades, axotomy and various nerve injury models have been used to study associations between pain-related behaviors and neurochemical changes in, for example, neuropeptide expression at the spinal level. However, from a clinical point of view, bone fracture is highly relevant, especially in the growing elderly population. In the present study, we compared a model of orthopedic surgery with a classical nerve injury (axotomy, complete transection of the sciatic nerve) and evaluated changes in pain behavior up to 2 wk after experimental intervention. Expression of a variety of pain-related and other markers was also analyzed in the somatosensory system and the hippocampal formation using IHC and ISH.
The main findings of this study are that tibial fracture with pinning causes the following: (i) cold allodynia and changes in nerve-injury markers at the spinal level [these changes recapitulate, although to a lesser degree, those seen in the parallel experiments with axotomy. This is likely correlated with the extent of nerve damage to the axons of DRG neurons. Moreover, these findings suggest that, for example, various “pain protective” and/or “regenerative” roles described, for example, for galanin and NPY, in response to nerve injury (36) also are active after tibial fracture]; (ii) expression of BDNF in DRG neurons after tibial fracture remaining elevated after 2 wk, versus having decreased in the axotomy model at this time point; (iii) acute activation of c-Fos in the spinal dorsal horn but decreased c-Fos levels in the hippocampal dentate gyrus; and (iv) a strong and acute increase of BDNF protein levels in hippocampal mossy fibers, paralleled by unchanged Bdnf mRNA in the parent granule cell bodies and a pronounced decrease in staining for DCX, a microtubule-associated protein expressed by neuronal precursor cells and used as a marker for neurogenesis (37). This contrasts with the lack of effect on expression levels of four neuropeptides (dynorphin, NPY, CCK, enkephalin) partly colocalized in the same mossy fibers/granule cells. We interpret the results under (iii) and (iv) to suggest an acute effect of bone fracture on BDNF signaling, neurogenesis, and cognitive processes.
The circuitry involved remains to be defined. An example of a possible pathway from the spinal cord to the forebrain relaying pain could include the paragigantocellular nucleus (38) and the noradrenergic locus coeruleus (39), which widely projects to forebrain areas, including the hippocampal formation (40). Noradrenergic mechanisms in pain modulation have been thoroughly explored (41, 42).
Development of Cold Allodynia.
Postoperative pain is a major clinical problem with a profound negative impact on patient recovery (43). The prevalence of postoperative pain significantly varies according to different procedures; after total hip or knee arthroplasty, ∼6% of patients experience neuropathic, chronic pain (44).
In this study, we investigated an effect of tibial surgery on three different pain-related behaviors, but were only able to detect cold allodynia during the relatively short period studied. This type of allodynia has been described in animal pain models (45–47) and also in patients after hand fracture (48, 49). However, the underlying mechanism(s) of cold allodynia in our model remains unclear.
In other orthopedic surgery animal models (i.e., femoral pin placement and fracture or fracture with casting), spontaneous, palpation-induced nocifensive behaviors and mechanical allodynia were observed (50, 51), contrasting our results where mice did not display secondary tactile allodynia after tibial fracture with intramedullary pinning. For example, Guo et al. (51) reported mechanical allodynia in rats using von Frey filaments after distal tibial fracture with a cast, but only monitored behavior at a chronic stage (4 wk after surgery). For nocifensive behavior in a femoral pin placement and fracture model, Majuta et al. (50) gently and repeatedly pressed, with thumb and forefinger, the thigh of the fractured hind limb for 2 min. This is different from our protocol of carrying out the von Frey filament test no later than 2 wk after fracture, when the leg is not completely stable (50, 52, 53).
Regulation of Injury-Sensitive Molecules in DRGs.
ATF3 is a classical marker of nerve injury, as first reported by Tsujino et al. (54). Hill et al. (55) reported that just skin incision, without substantial nerve damage, causes an increase of ATF3, galanin, and other injury markers; ATF3 is found in 2.9% of DRG neurons after skin incision vs. 0.07% in controls. Up-regulation of such markers has been described in multiple conditions, including osteoarthritis, chemotherapy, and other noninvasive procedures, although osteoarthritis and chemotherapy are likely to damage nerves (56–60).
Functionally, ATF3 has been reported to enhance peripheral nerve regeneration (61). ATF3 can also be activated in both DRG neurons and satellite cells after i.v. administration of paclitaxel, an antineoplastic drug (57, 62). In the latter studies, ATF3 is first activated only in DRG neurons and then in clustered satellite cells, similar to so-called nodules of Nageotte, which represent neuronal degeneration/cell loss in DRGs (62). Here we show that bone fracture also induces ATF3 expression in low numbers of Schwann cells and satellite cells located in the axon trunks within the ganglion, without clustering; however, these effects are much more pronounced after axotomy.
The neuropeptide galanin (63), acting via three receptors, Gal1–3 (64, 65), has also emerged as an injury marker and as an “endogenous analgesic” (66). Galanin shows a dramatic up-regulation in DRG neurons, mainly in the small- and medium-sized population (67, 68), after transection of the sciatic nerve and, as shown here, to a lesser extent following tibial fracture. The apparently transient elevation after fracture may suggest that the antinociceptive effect of this peptide via the Gal1 subtype receptor (69) also is of limited duration and could contribute to extended pain sensation. Comprehensive studies by the Wynick group have reported a trophic role of galanin via the Gal2 receptor (70). Galanin has also been implicated in cold allodynia, as found in our study. Intrathecal infusion of a Gal2/3 agonist can in fact augment the nociceptive response to acetone (69). Moreover, Gal1-KO mice exhibit increased cold pain sensitivity (71), also indicating involvement of this receptor, supported by a comprehensive study by Hulse et al. (72). Thus, peripheral administration of galanin, but not of a Gal2/3 agonist, inhibits pain induced by acetone and menthol in neuropathic and inflammatory models, as does overexpression of galanin in DRG neurons.
NPY (73) is not expressed in DRG neurons under normal circumstances but is expressed abundantly in dorsal horn neurons (74). However, nerve injury causes up-regulation in mainly large, DRG neurons (75). There is evidence for involvement of NPY in cold sensation (46, 76); for example, NPY reduces cold hypersensitivity (77), and NPY is one of eight genes that are regulated in DRGs by repeated cold stress (78).
BDNF (79) modulates a wide variety of functions in the peripheral nervous system, including pain (80, 81), and has a considerable therapeutic potential (82). It is mainly expressed in small- and medium-sized DRG neurons (83). BDNF is increased in DRGs and spinal cord following peripheral inflammation, and is in this situation considered to have pronociceptive effects at the spinal level (84, 85). It is up-regulated by nerve injury in rat DRGs (26), but also shows down-regulation upon long-term nerve injury in neonatal rat DRGs (86). In contrast to inflammatory conditions, if BDNF levels are increased by nerve injury (neuropathic pain) or by exogenous administration/viral overexpression in the spinal cord, then this growth factor has antinociceptive effects (87, 88).
Here we report that BDNF is up-regulated after axotomy during the early phase of nerve injury but returns back to normal levels after 2 wk, in fact even below basal levels. However, expression of BDNF after tibial fracture remains elevated after 2 wk. Whether or not BDNF is involved in cold sensation after tibial fracture still needs further investigation.
Modulation of BDNF in the Hippocampal Formation.
BDNF is a key signaling molecule involved in a wide range of central functions and neuronal plasticity, including modulatory actions in the hippocampus of relevance to learning, memory, neurogenesis, and mood control (89–93). Memory impairment after surgery has also been associated with reduced BDNF expression (94–97). Conditional knockout of BDNF in the forebrain impairs hippocampal-dependent learning in mice (98, 99). However, BDNF overexpression also results in working memory deficits, increased anxiety-like traits, and seizure susceptibility (100).
Here we show distinct BDNF levels in mossy fibers, using two independent antibodies, confirming earlier findings (32, 34, 101), as well as a marked increase after tibial fracture. Surprisingly, this robust increase of BDNF protein in mossy fibers (IHC) is not paralleled by elevated Bdnf mRNA levels in granule cell bodies giving rise to mossy fibers (ISH and RT-qPCR). This contrasts with the results in several other brain regions, such as the amygdala, where we found increased levels of both BDNF protein and Bdnf mRNA in the basolateral amygdaloid nucleus, as well as increased levels of BDNF-LI in nerve terminals in the central amygdala. At least some of these nerve endings may belong to the projection from the basolateral nucleus (102), but the central nucleus also receives input from other brain regions, such as the parabrachial nucleus (103). Thus, a connection between the cell bodies and nerve endings (shown in Fig. 5F, F4 and F5, respectively) remains to be established. Nevertheless, this may represent a projection, where BDNF is differently regulated compared with the dentate gyrus: The increase in protein is paralleled by an increased transcript level. This would support the idea that the lack of effect on mRNA levels in the granule cell layer after fracture is not an artifact but a unique reaction for hippocampal granular (and other) cells.
It has now been definitively shown that BDNF in mossy fibers is stored in large dense core vesicles (LDCVs) (34) that represent the organelle from which this molecule and neuropeptides in general are released. Our results confirm many previous studies showing that the three peptides enkephalin (104), dynorphin (104), and CCK (105) are expressed in mossy fibers, and also that NPY is not detectable under normal circumstances (106). Three of these are strongly increased in epilepsy models, whereas CCK is down-regulated (34, 35, 107). Dieni et al. (34) have shown that CCK or met-enkephalin can be stored in the same granule cells/mossy fibers as BDNF, but not in the same LDCVs. Our results show that bone fracture apparently is not a sufficiently strong stimulus to modulate neuropeptide expression or, alternatively, that a different circuitry is involved. Taken together, BDNF and neuropeptide molecules synthesized in the same granule cells can be directed into different LDCVs and are thus likely separately regulated and can be individually released.
In a pioneering study, Thoenen et al. (108) reported that the enzyme tyrosine hydroxylase in the adrenal medulla can be transsynaptically induced. Similarly, NPY (and dopamine β-hydroxylase) levels are dramatically and acutely increased in sympathetic ganglion cells by preganglionic electrical stimulation (109). With regard to peptides, a possible explanation is that the released peptide has to be compensated by increased synthesis, which is reflected by elevated mRNA levels. A similar principle may apply to BDNF. However, in the present experiments, the increased BDNF levels in mossy fibers are not paralleled by increased Bdnf mRNA levels. One possible interpretation is reduced BDNF release, hypothetically leading to acute cognitive problems, as previously described with this model (110). Reduced BDNF release from mossy fibers has, to our knowledge, not been previously demonstrated and, if true, the underlying mechanism(s) is unknown. Our hypothesis of reduced BDNF release is in line with the concept that increased BDNF levels augment neurogenesis (111, 112); thus, conversely, attenuated BDNF release/signaling should reduce neurogenesis in the dentate subgranular layer, as noted in our model. This is also consistent with our finding of decreased c-Fos expression, as c-Fos and BDNF are known to be coregulated (113–115), as well as with our previous data demonstrating impaired long-term potentiation 24 h after tibial surgery (110), tentatively indicating a transient effect of surgery on neuronal hyperexcitability. However, alternative explanations should be considered, such as posttranslational regulation of BDNF synthesis (116), and synthesis by already-existing BDNF mRNA cannot be excluded.
Other groups have used various cognitive tests and associated postoperative dysfunction with BDNF signaling, but these groups reported decreased BDNF protein levels (95, 96, 117, 118). Even if contrasting our increase in BDNF protein, the functional consequence is the same as ours. Here are some relevant excerpts from these studies: Fidalgo et al. (96) used an intramedullary pin in tibia in mice and recorded decreased BDNF levels in the hippocampus with Western blotting; Hovens et al. (117) used immunohistochemistry and found decreased BDNF levels after 2 and 3 (but not 1) wk in the pyramidal cell layer of rats; Tian et al. (95) carried out abdominal surgery and found decreased BDNF levels in the prefrontal cortex with Western blotting, but only in old mice, and only after 20 mo (not after 6 mo); and Fan et al. (118) exposed the carotid artery of mice and found decreased BDNF levels using ELISA 5 d after surgery. They also provided evidence for decreased neurogenesis, in agreement with our findings. In summary, these four and our studies are characterized by a number of distinct differences that may explain apparently conflicting results: species, injury model, analysis method, and time course.
Nerve Injury, Pain, and Cognition.
The focus in this study is on neurochemical changes at the spinal level and in the hippocampal formation after bone fracture. The possible relationships between surgery/nerve injury, pain, and cognition are complex (6–13) and involve the hippocampus (12, 119, 120). Moreover, patients already suffering from cognitive deficits are at greater risk for pain chronicity after a painful event, including surgical manipulations (121). This vulnerability may have implications for postoperative recovery, thus predisposing for complications such as delirium and postoperative cognitive dysfunction. These are complex multifactorial mechanisms [recently reviewed in Berger et al. (122)], and a better understanding of the pain-related signatures after orthopedic surgery may lead to novel strategies to prevent these complications. However, some evidence does not support long-term cognitive deficits from surgery in humans (123, 124), and rodent models to date are limited in differentiating between acute delirium and longer-lasting cognitive decline. It should be also noted that important sex differences have been described in pain research (125, 126), and our future studies should take this into account. Overall, because of the differential role of BDNF described in our model and the importance of this molecule in both pain signaling and memory function (89, 127), further studies are warranted to ascertain the circuitry underlying nerve injury, pain, and cognitive impairment.
Materials and Methods
Animals.
Wild-type C57BL/6 mice (adult male, 12–14 wk of age) and Cx3cr1GFP/+ knockin mice (128) (adult male, 12 wk of age) were included in this study (SI Materials and Methods). The experiments were conducted in accordance with Swedish policy for the use of research animals and were approved by a local ethical committee (Stockholms Norra djurförsöksetiska nämnd) and the Institutional Animal Care and Use Committee of Duke University (protocol A120-15-04).
Surgery.
Tibial fracture surgery with intramedullary pinning was performed essentially as described (22, 110). Complete transection of the sciatic nerve (axotomy) was performed as previously described (129, 130) (SI Materials and Methods).
Behavior Tests.
Withdrawal threshold was tested in transparent plastic domes on a metal mesh floor and measured by a logarithmically incremental stiffness of 0.04, 0.07, 0.16, 0.40, 0.60, 1.0, and 2.0 (g) von Frey Filament (Stoelting) combined with an up–down method to assess tactile allodynia (25, 52, 53). The cutoff of a 2.0 hair was selected as the upper limit for testing. For mechanical hyperalgesia, a safety pin was used, and the duration of paw withdrawal was recorded (25, 131). Cold allodynia was tested with a drop of acetone, and the duration of the withdrawal response was recorded (25, 131).
Tissues.
For IHC, mice were deeply anesthetized, perfused transcardially with 4% (wt/vol) paraformaldehyde containing picric acid, and processed for staining as previously described (130, 132). For ISH, mice were deeply anesthetized and brains were removed immediately and stored in a −80 °C freezer after sacrifice (SI Materials and Methods).
In Situ Hybridization.
Plasmid DNA containing RNA probes specific for mouse Bdnf was provided by P. Ernfors, Karolinska Institutet. ISH was performed as previously described (133) (SI Materials and Methods).
RT-qPCR.
Quantitative PCR was run on a StepOnePlus Real-Time PCR System (Applied Biosystems) (SI Materials and Methods).
Immunohistochemistry.
Sections were dried at room temperature for at least 30 min and then incubated with primary antibodies (Table S2) diluted in PBS containing 0.2% (wt/vol) BSA (Sigma) and 0.03% Triton X-100 (Sigma) in a humid chamber at 4 °C for 48 h. Immunoreactivities were visualized using the TSA Plus Kit (PerkinElmer) as previously described (130, 132) (SI Materials and Methods). Of note, all micrographs showing BDNF staining are based on the Amgen antibody (Table S2).
Table S2.
Primary antibodies used for immunohistochemistry in this study
| Antibody | Host | Antigen | Supplier/catalog no. | Dilutions |
| ATF3 | Rabbit/polyclonal | C-terminal sequence of human ATF3 (amino acids 194–212) | Santa Cruz/sc-188 | 1:4,000 tsa |
| BDNF | Rabbit/polyclonal | Recombinant human BDNF covalently coupled to KLH | Amgen | 1:2,000 tsa |
| BDNF | Mouse/monoclonal | Fish and human mature BDNF | Y.-A. Barde, Cardiff University, Cardiff, UK | 1:1,000 tsa |
| c-Fos | Rabbit/polyclonal | Synthetic peptide corresponding to human amino acids 4–17 | Calbiochem/PC38 | 1:2,000 tsa |
| CCK | Rabbit/polyclonal | Nonsulfated CCK8 coupled to KLH | P. Frey, Sandoz Research Institute, Bern, Switzerland | 1:8,000 tsa |
| Dyn | Rabbit/polyclonal | Synthetic peptide corresponding to dynorphin A | G. Bakalkin, Uppsala University, Uppsala, Sweden | 1:4,000 tsa |
| ENK | Rabbit/polyclonal | Synthetic met-enkephalin coupled to BSA | 1:4,000 tsa | |
| Galanin | Rabbit/polyclonal | Synthetic peptide corresponding to amino acids 1–29 of rat galanin | E. Theodorsson, Linköping University, Linköping, Sweden | 1:4,000 tsa |
| GFAP | Rabbit/polyclonal | GFAP from bovine spinal cord | DAKO/Z0334 | 1:1,000 |
| Iba1 | Rabbit/polyclonal | Conserved C-terminal synthetic peptides | Wako/019-19741 | 1:500 |
| NPY | Rabbit/polyclonal | Synthetic peptide corresponding to full-length porcine NPY | H. Wong and J. H. Walsh, UCLA, Los Angeles, CA | 1:4,000 tsa |
KLH, keyhole limpet hemocyanin.
Imaging and Analysis.
Representative images were acquired from one airy unit pinhole on an LSM 700 confocal laser-scanning microscope (Carl Zeiss). Multipanel figures were assembled using Photoshop CS6 software (Adobe Systems). Quantification and intensity analysis were performed using Adobe Photoshop CS6 software manually and ImageJ version 1.46 software (National Institutes of Health) (SI Materials and Methods).
Statistics.
The behavior data of the von Frey filament test (nonparametric values) are presented as median with range and assessed by Mann–Whitney test. Behavior data of pinprick and acetone tests, data on immunohistochemistry, and RT-qPCR are presented as mean ± SD or mean ± SEM and analyzed with unpaired t test or one-way ANOVA for different time points as indicated in the figure legends by using Prism 6 software (GraphPad Software). The criterion for statistical significance was P < 0.05.
SI Materials and Methods
Animals.
Wild-type C57BL/6 mice (adult male, 12–14 wk of age) and Cx3cr1GFP/+ knockin mice (128) (adult male, 12 wk of age) were included in this study. Mice were kept under standard conditions on a 12/12-h light/dark cycle with free access to food and water. The experiments were conducted in accordance with Swedish policy for the use of research animals and were approved by a local ethical committee (Stockholms Norra djurförsöksetiska nämnd) and the Institutional Animal Care and Use Committee of Duke University (protocol A120-15-04). Mice were acclimated ≥1 wk before starting any experiment and checked on a daily basis for overall appearance and body condition. Efforts were made to minimize the number of mice used and their suffering throughout.
Surgery.
Tibial fracture surgery was performed essentially as described previously (22, 110). The open stabilized tibial fracture procedure was performed under isoflurane anesthesia (2.1% inspired concentration in 0.30 FiO2) and analgesia (buprenorphine, 0.1 mg/kg s.c.). Briefly, a longitudinal incision was performed on the left hind paw under strictly aseptic conditions. Muscles were disassociated, and a 0.38-mm stainless steel pin was inserted into the upper crest of the tibia, into the intramedullary canal. Osteotomy was then performed, and the wound was irrigated and sutured with 6-0 Prolene. Mice were allowed to recover in a warm box before returning to the home cage. Pulse oximetry was continuously recorded (Kent Scientific) and temperature was monitored and maintained at 37 °C with the aid of a warming pad and temperature-controlled lights (Harvard Apparatus). The mice were allowed to survive for 2 h, 6 h, 24 h, 3 d, and 2 wk, and the sham group for 3 d. In the sham group, a longitudinal incision was performed on the left hind paw under strictly aseptic conditions and the muscles were disassociated. However, no pin was inserted and no fracture was carried out. This is because pinning also requires a fracturing of the bone, although only minor, to insert the metal rod. Complete transection of the sciatic nerve (axotomy) was performed as previously described (129, 130). The mice were allowed to survive for 6 h, 24 h, 3 d, and 2 wk. Mice without any surgery were also included for tibial fracture and axotomy experiments as controls.
Tissues.
For immunohistochemistry, control mice and mice undergoing surgery were deeply anesthetized with sodium pentobarbital (50 mg/kg administered i.p.) and perfused transcardially with 4% paraformaldehyde containing picric acid as previously described (130, 132). The lumbar (L4 and L5) dorsal root ganglia (DRGs) and the corresponding lumbar segments of the spinal cord and brain were dissected out and postfixed in the same fixative for 90 min at 4 °C, followed by rinsing in 10% (wt/vol) sucrose in phosphate buffer (0.1 M, pH 7.2) containing 0.01% sodium azide (Merck) and 0.02% bacitracin (Sigma). The tissues were kept in 10% sucrose solution for 2 d at 4 °C. All trimmed tissues were embedded with optimal cutting temperature compound (Histolab), frozen with liquid carbon dioxide, and sectioned on an NX70 cryostat (Thermo Scientific) at a thickness of 12 μm for DRGs and 20 μm for the spinal cord and brain. The sections were mounted onto Superfrost Plus microscope slides (Thermo Scientific) and stored at −20 °C.
For in situ hybridization, mice with tibial fracture for 2 h, 6 h, and 24 h, sham group for 24 h, and control mice were deeply anesthetized with sodium pentobarbital and brains were removed immediately and stored in a −80 °C freezer after sacrifice. Sections (20-μm) were prepared before the experiment.
Riboprobe Synthesis for in Situ Hybridization.
Plasmid DNA containing RNA probes specific for mouse Bdnf was provided by Patrik Ernfors, Karolinska Institutet. After linearization of the plasmid DNA, antisense and sense riboprobes were transcribed by T7/Sp6 RNA polymerase (Life Technologies) in the presence of [35S]UTP (PerkinElmer). Unincorporated nucleotides were removed on NucAway spin columns (Life Technologies).
In Situ Hybridization.
In situ hybridization was performed as previously described (133), with minor modifications. Briefly, hippocampal sections were postfixed in 4% paraformaldehyde and then treated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0). The sections were dehydrated in graded alcohol and stored at −20 °C. Sections were prehybridized using 50% (vol/vol) deionized formamide (pH 5.0), 50 mM Tris⋅HCl (pH 7.6), 25 mM EDTA (pH 8.0), 20 mM NaCl, 0.25 mg/mL yeast tRNA, and 2.5× Denhardt’s solution for 4 h at 55 °C followed by hybridization in a humidified chamber overnight (14–16 h) at 55 °C. Labeled probes were diluted to a final concentration of 1.0 × 106 cpm per 200 μL in a solution containing 50% (vol/vol) deionized formamide (pH 5.0), 0.3 M NaCl, 20 mM DTT, 0.5 mg/mL yeast tRNA, 0.1 mg/mL poly(A) RNA, 10% (vol/vol) dextran sulfate, and 1× Denhardt’s solution. After hybridization, sections were washed twice for 30 min in 1× SSC at 55 °C, 1 h in 50% (vol/vol) formamide/0.5× SSC at 55 °C, 15 min in 1× SSC at 55 °C, 1 h in RNase A buffer at 37 °C, twice for 15 min in 1× SSC at 55 °C, dehydrated in an ascending series of ethanol (70%, 90%, 95%, 100%; 2 min each), and air-dried. Sections were first exposed to Kodak BioMax MR film for 24 h (VWR International) and then dipped in an autoradiographic emulsion (Kodak). After exposing the slides for 48 h, they were developed using D19 developer (Kodak) for 3 min and AL4 fixative (Kodak) for 7 min, dried at room temperature (RT; 22–24 °C), and then mounted with a medium containing 90% glycerol/10% PBS (vol/vol).
RT-qPCR.
Mice were anesthetized with isoflurane and perfused transcardially with 30 mL of normal saline. After brains were removed, hippocampi were dissected and flash-frozen in liquid nitrogen and then stored at −80 °C until RNA extraction. RNA was extracted using an RNA Lipid Tissue Mini Kit (Qiagen) according to the manufacturer’s manual, and quantitated with an ND-1000 spectrophotometer (NanoDrop Technologies). First-strand cDNA was generated from 400 ng of total RNA in 20 µL using qScript cDNA SuperMix (Quanta Biosciences). One microliter of cDNA template was applied in each 20 µL PCR with Power SYBR Green PCR Master Mix (Applied Biosystems). Quantitative PCR was run on a StepOnePlus Real-Time PCR System (Applied Biosystems) for 10 min at 95 °C and then 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Primer sequences of mouse Bdnf were:
Forward: 5′-GTGACTGAAAAAGTTCCACC-3′;
Reverse: 5′-GACGTTTACTTCTTTCATGGG-3′.
Primer sequences for mouse β-actin were:
Forward: 5′-GGACCTGACGGACTACCTCATG-3′;
Reverse: 5′-TCTTTGATGTCACGCACGATTT-3′.
Immunohistochemistry.
Sections were dried at RT for at least 30 min and then incubated with primary antibodies (Table S2) diluted in PBS containing 0.2% (wt/vol) BSA (Sigma) and 0.03% Triton X-100 (Sigma) in a humid chamber at 4 °C for 48 h. Immunoreactivities were visualized using the TSA Plus Kit (PerkinElmer) as previously described (130, 132). The slides were counterstained with 0.001% propidium iodide (PI; Sigma) and covered with 1,4-diazabicyclo(2.2.2)octane (DABCO, Sigma). For the staining of Iba1 or GFAP on DRGs and spinal cord from Cx3cr1GFP/+ knockin mice, selected sections were dried, rinsed in PBS, and incubated with primary antibodies for 48 h at 4 °C. The glial staining was developed with Cy5-conjugated, affinity-purified donkey anti-rabbit IgG (1:100; Jackson ImmunoResearch Laboratories) at RT for 90 min and mounted in DABCO medium.
Imaging and Analysis.
Representative images were acquired from one airy unit pinhole on an LSM 700 confocal laser-scanning microscope (Carl Zeiss) equipped with an objective with an EC Plan-Neofluar objective with a magnification of 5× and 10× and N.A. of 0.30, a Plan-Apochromat M27 objective with a magnification of 20× and N.A. of 0.80, and a water objective with a magnification of 40× and N.A. of 1.40. Emission spectra for each dye were limited as follows: FITC (505–540 nm), Cy3/PI (560–610 nm), and Cy5 (>640 nm). In some cases, orthogonal z stacks were acquired with a depth interval of 2 μm with an objective with a magnification of 20×, or 1 μm with an objective with a magnification of 40× (as indicated in the figure legends). Images were processed using ZEN 2012 software (Zeiss). Dark-field pictures of in situ hybridization were captured on a Nikon microscope equipped with a Coolpix 5000 digital camera (Nikon). Multipanel figures were assembled using Photoshop CS6 software (Adobe Systems).
For quantification of neuron profiles (NPs) of markers in DRG neurons, two to four L4/L5 DRG sections counterstained with PI were selected from different levels (usually a four-section interval) and tile-scanned with an LSM 700 laser-scanning microscope equipped with a Plan-Apochromat M27 objective with a magnification of 20× and N.A. of 0.80. The intensity of marker-like immunoreactivities in neurons higher than mean plus twofold SD of the soma of negative neurons from each section was considered positive. The total number of DRG NPs was counted on PI-stained sections. All of the counting was performed using Adobe Photoshop CS6 software manually.
The intensity of Iba1-LI in DRGs and BDNF-LI in the hippocampus was analyzed with ImageJ version 1.46 software (National Institutes of Health) on the tile-scanned pictures (20× M27 objective for DRG, and 10× objective for hippocampus; LSM 700). Iba1 labels macrophages/microglia in DRGs, and their intensity was calculated as mean gray value after subtraction of background. The mean gray value of BDNF intensity was measured in the polymorph layer of the dentate gyrus (PoDG) and stratum lucidum (SLu) of CA3 from hippocampus.
For quantification of Bdnf signals from in situ hybridization, the expression levels of Bdnf mRNA in the CA1, CA3, and dentate gyrus cell clusters of controls and the three fracture groups were quantified using an image analysis system including a Nikon Eclipse E600 microscope (equipped with a dark-field Heim fiber optic condenser; LQ1600), digital camera (ORCA-ER, C4742-80; Hamamatsu Photonics), and Hamamatsu Photonics Wasabi 150 software. The density of silver grains was measured with ImageJ software version 1.37 (NIH). For analysis, all labeled cells were analyzed on two micrographs from each section (bilaterally). The threshold was adjusted to the background signal level, and the “mean gray density” was determined with respect to the surface area of the selected regions.
Acknowledgments
We thank Prof. P. Ernfros (Department of Biochemistry and Biophysics, Karolinska Institutet) for generously providing the plasmid for BDNF; Prof. M. Sendtner (Institute for Clinical Neurobiology, University of Würzburg) for reagents, advice, and comments; Profs. B. Hempstead (Weill Cornell Medical College), E. Castrén (Neuroscience Center, University of Helsinki), and R.-R. Ji (Duke University Medical Center) for valuable advice and comments; and, especially, Prof. Y.-A. Barde (School of Biosciences, Cardiff University) for critically reading the manuscript and providing valuable suggestions for new experiments and ideas for the discussion. We thank Profs. G. Bakalkin (dynorphin, Uppsala University), P. Frey (CCK, Sandoz Research Institute, Bern), E. Theodorsson (galanin, Linköping University), and late H. Wong and J. H. Walsh (NPY, UCLA) for general donation of antisera. Support for this study was provided by the Swedish Medical Research Council (T.G.M.H. and T.H.), Karolinska Institutet partial financing of graduate student funds (M.-D.Z., T.H., and T.G.M.H.); funding from NIH/National Institute of Neurological Disorders and Stroke NS052189, NS051470, and NS082976 (to K.A.), Karolinska Institutet (T.G.M.H., T.H., and N.T.), Novo Nordisk Foundation (T.H. and T.G.M.H.), Swedish Brain Foundation (T.H. and T.G.M.H.), Augusta and Petrus Hedlund Foundation (T.H. and T.G.M.H.), European Commission’s PAINCAGE Seventh Framework Programme integrated project (T.H. and T.G.M.H.), European Society of Anesthesiology, and Duke University Anesthesiology Department (N.T.). Laser-scanning microscopy was made available by the Center for Live Imaging of Cells at Karolinska Institutet, an imaging core facility supported by the Knut and Alice Wallenberg Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614017113/-/DCSupplemental.
References
- 1.Munch T, et al. Pain and falls and fractures in community-dwelling older men. Age Ageing. 2015;44(6):973–979. doi: 10.1093/ageing/afv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roche JJ, Wenn RT, Sahota O, Moran CG. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: Prospective observational cohort study. BMJ. 2005;331(7529):1374. doi: 10.1136/bmj.38643.663843.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: Tailoring care for the older patient. JAMA. 2012;307(20):2185–2194. doi: 10.1001/jama.2012.4842. [DOI] [PubMed] [Google Scholar]
- 4.Fong HK, Sands LP, Leung JM. The role of postoperative analgesia in delirium and cognitive decline in elderly patients: A systematic review. Anesth Analg. 2006;102(4):1255–1266. doi: 10.1213/01.ane.0000198602.29716.53. [DOI] [PubMed] [Google Scholar]
- 5.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: A randomized trial. J Am Geriatr Soc. 2001;49(5):516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 6.Moller JT, et al. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet. 1998;351(9106):857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 7.Ehlenbach WJ, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog Neurobiol. 2011;93(3):385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Hart RP, Martelli MF, Zasler ND. Chronic pain and neuropsychological functioning. Neuropsychol Rev. 2000;10(3):131–149. doi: 10.1023/a:1009020914358. [DOI] [PubMed] [Google Scholar]
- 10.Ren WJ, et al. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents. Neuropsychopharmacology. 2011;36(5):979–992. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman ME, et al. Hippocampal correlates of pain in healthy elderly adults: A pilot study. Neurology. 2009;73(19):1567–1570. doi: 10.1212/WNL.0b013e3181c0d454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutso AA, et al. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32(17):5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kline RP, et al. Surgery and brain atrophy in cognitively normal elderly subjects and subjects diagnosed with mild cognitive impairment. Anesthesiology. 2012;116(3):603–612. doi: 10.1097/ALN.0b013e318246ec0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harry LE, et al. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res. 2008;26(9):1238–1244. doi: 10.1002/jor.20649. [DOI] [PubMed] [Google Scholar]
- 15.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 16.Staff NP, et al. Post-surgical inflammatory neuropathy. Brain. 2010;133(10):2866–2880. doi: 10.1093/brain/awq252. [DOI] [PubMed] [Google Scholar]
- 17.Terrando N, et al. Perioperative cognitive decline in the aging population. Mayo Clin Proc. 2011;86(9):885–893. doi: 10.4065/mcp.2011.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabsovich I, et al. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138(1):47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCaffrey G, et al. NGF blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer Res. 2014;74(23):7014–7023. doi: 10.1158/0008-5472.CAN-14-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabsovich I, et al. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain. 2008;137(3):507–519. doi: 10.1016/j.pain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li WW, et al. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain. 2009;144(3):303–313. doi: 10.1016/j.pain.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terrando N, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci USA. 2010;107(47):20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terrando N, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu SM, et al. S100A8 contributes to postoperative cognitive dysfunction in mice undergoing tibial fracture surgery by activating the TLR4/MyD88 pathway. Brain Behav Immun. 2015;44:221–234. doi: 10.1016/j.bbi.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Decosterd I, Woolf CJ. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2):149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 26.Michael GJ, Averill S, Shortland PJ, Yan Q, Priestley JV. Axotomy results in major changes in BDNF expression by dorsal root ganglion cells: BDNF expression in large trkB and trkC cells, in pericellular baskets, and in projections to deep dorsal horn and dorsal column nuclei. Eur J Neurosci. 1999;11(10):3539–3551. doi: 10.1046/j.1460-9568.1999.00767.x. [DOI] [PubMed] [Google Scholar]
- 27.Souza GR, et al. Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci USA. 2013;110(27):11193–11198. doi: 10.1073/pnas.1307445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verge GM, et al. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20(5):1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 29.Curran T, Morgan JI. Fos: An immediate-early transcription factor in neurons. J Neurobiol. 1995;26(3):403–412. doi: 10.1002/neu.480260312. [DOI] [PubMed] [Google Scholar]
- 30.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328(6131):632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 31.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 32.Yan Q, et al. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78(2):431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- 33.Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5(4):511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 34.Dieni S, et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196(6):775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasterlain CG, et al. Short-term plasticity of hippocampal neuropeptides and neuronal circuitry in experimental status epilepticus. Epilepsia. 2002;43(Suppl 5):20–29. doi: 10.1046/j.1528-1157.43.s.5.1.x. [DOI] [PubMed] [Google Scholar]
- 36.Brumovsky P, Shi TS, Landry M, Villar MJ, Hokfelt T. Neuropeptide tyrosine and pain. Trends Pharmacol Sci. 2007;28(2):93–102. doi: 10.1016/j.tips.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Couillard-Despres S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21(1):1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 38.Aston-Jones G, et al. Afferent regulation of locus coeruleus neurons: Anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- 39.Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E. Noradrenergic locus coeruleus pathways in pain modulation. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 40.Moore RY, Bloom FE. Central catecholamine neuron systems: Anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 41.Szabadi E. Modulation of physiological reflexes by pain: Role of the locus coeruleus. Front Integr Neurosci. 2012;6:94. doi: 10.3389/fnint.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80(2):53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Taylor RS, et al. The impact of early postoperative pain on health-related quality of life. Pain Pract. 2013;13(7):515–523. doi: 10.1111/papr.12026. [DOI] [PubMed] [Google Scholar]
- 44.Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: A systematic literature review. Pain. 2013;154(1):95–102. doi: 10.1016/j.pain.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59(3):369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 46.Datta S, Chatterjee K, Kline RH, IV, Wiley RG. Behavioral and anatomical characterization of the bilateral sciatic nerve chronic constriction (bCCI) injury: Correlation of anatomic changes and responses to cold stimuli. Mol Pain. 2010;6:7. doi: 10.1186/1744-8069-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caspani O, Zurborg S, Labuz D, Heppenstall PA. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One. 2009;4(10):e7383. doi: 10.1371/journal.pone.0007383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serra J, et al. C-nociceptors sensitized to cold in a patient with small-fiber neuropathy and cold allodynia. Pain. 2009;147(1–3):46–53. doi: 10.1016/j.pain.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 49.Smits ES, et al. Rewarming patterns in hand fracture patients with and without cold intolerance. J Hand Surg Am. 2011;36(4):670–676. doi: 10.1016/j.jhsa.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 50.Majuta LA, Longo G, Fealk MN, McCaffrey G, Mantyh PW. Orthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factor. Pain. 2015;156(1):157–165. doi: 10.1016/j.pain.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108(1–2):95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 53.Bas DB, et al. Collagen antibody-induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency. Arthritis Rheum. 2012;64(12):3886–3896. doi: 10.1002/art.37686. [DOI] [PubMed] [Google Scholar]
- 54.Tsujino H, et al. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15(2):170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- 55.Hill CE, et al. Skin incision induces expression of axonal regeneration-related genes in adult rat spinal sensory neurons. J Pain. 2010;11(11):1066–1073. doi: 10.1016/j.jpain.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calza L, et al. Peptide plasticity in primary sensory neurons and spinal cord during adjuvant-induced arthritis in the rat: An immunocytochemical and in situ hybridization study. Neuroscience. 1998;82(2):575–589. doi: 10.1016/s0306-4522(97)00272-8. [DOI] [PubMed] [Google Scholar]
- 57.Peters CM, et al. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol. 2007;203(1):42–54. doi: 10.1016/j.expneurol.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 58.Ivanavicius SP, et al. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: Increased expression of ATF-3 and pharmacological characterisation. Pain. 2007;128(3):272–282. doi: 10.1016/j.pain.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 59.Su J, et al. Phenotypic changes in dorsal root ganglion and spinal cord in the collagen antibody-induced arthritis mouse model. J Comp Neurol. 2015;523(10):1505–1528. doi: 10.1002/cne.23749. [DOI] [PubMed] [Google Scholar]
- 60.Park HJ, Stokes JA, Corr M, Yaksh TL. Toll-like receptor signaling regulates cisplatin-induced mechanical allodynia in mice. Cancer Chemother Pharmacol. 2014;73(1):25–34. doi: 10.1007/s00280-013-2304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27(30):7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, Mantyh PW. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. 2007;1168:46–59. doi: 10.1016/j.brainres.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V. Galanin—A novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 64.Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21(3):109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- 65.Habert-Ortoli E, Amiranoff B, Loquet I, Laburthe M, Mayaux JF. Molecular cloning of a functional human galanin receptor. Proc Natl Acad Sci USA. 1994;91(21):9780–9783. doi: 10.1073/pnas.91.21.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu XJ, Hokfelt T, Wiesenfeld-Hallin Z. Galanin and spinal pain mechanisms: Past, present, and future. EXS. 2010;102:39–50. doi: 10.1007/978-3-0346-0228-0_4. [DOI] [PubMed] [Google Scholar]
- 67.Hokfelt T, Wiesenfeld-Hallin Z, Villar M, Melander T. Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci Lett. 1987;83(3):217–220. doi: 10.1016/0304-3940(87)90088-7. [DOI] [PubMed] [Google Scholar]
- 68.Villar MJ, et al. Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience. 1989;33(3):587–604. doi: 10.1016/0306-4522(89)90411-9. [DOI] [PubMed] [Google Scholar]
- 69.Liu HX, et al. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: Selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci USA. 2001;98(17):9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hobson SA, et al. Galanin acts as a trophic factor to the central and peripheral nervous systems. EXS. 2010;102:25–38. doi: 10.1007/978-3-0346-0228-0_3. [DOI] [PubMed] [Google Scholar]
- 71.Blakeman KH, et al. Hyperalgesia and increased neuropathic pain-like response in mice lacking galanin receptor 1 receptors. Neuroscience. 2003;117(1):221–227. doi: 10.1016/s0306-4522(02)00779-0. [DOI] [PubMed] [Google Scholar]
- 72.Hulse RP, Donaldson LF, Wynick D. Differential roles of galanin on mechanical and cooling responses at the primary afferent nociceptor. Mol Pain. 2012;8:41. doi: 10.1186/1744-8069-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y—A novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296(5858):659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 74.Gibson SJ, et al. The distribution and origin of a novel brain peptide, neuropeptide Y, in the spinal cord of several mammals. J Comp Neurol. 1984;227(1):78–91. doi: 10.1002/cne.902270109. [DOI] [PubMed] [Google Scholar]
- 75.Wakisaka S, Kajander KC, Bennett GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett. 1991;124(2):200–203. doi: 10.1016/0304-3940(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 76.Soril LJ, Ramer LM, McPhail LT, Kaan TK, Ramer MS. Spinal brain-derived neurotrophic factor governs neuroplasticity and recovery from cold-hypersensitivity following dorsal rhizotomy. Pain. 2008;138(1):98–110. doi: 10.1016/j.pain.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Intondi AB, Dahlgren MN, Eilers MA, Taylor BK. Intrathecal neuropeptide Y reduces behavioral and molecular markers of inflammatory or neuropathic pain. Pain. 2008;137(2):352–365. doi: 10.1016/j.pain.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kozaki Y, et al. Peripheral gene expression profile of mechanical hyperalgesia induced by repeated cold stress in SHRSP5/Dmcr rats. J Physiol Sci. 2015;65(5):417–425. doi: 10.1007/s12576-015-0380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pezet S, McMahon SB. Neurotrophins: Mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 81.Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55(1):1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Lindsay RM. Therapeutic potential of the neurotrophins and neurotrophin-CNTF combinations in peripheral neuropathies and motor neuron diseases. Ciba Found Symp. 1996;196:39–48, discussion 48–53. [PubMed] [Google Scholar]
- 83.Wetmore C, Olson L. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J Comp Neurol. 1995;353(1):143–159. doi: 10.1002/cne.903530113. [DOI] [PubMed] [Google Scholar]
- 84.Thompson SW, Bennett DL, Kerr BJ, Bradbury EJ, McMahon SB. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci USA. 1999;96(14):7714–7718. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mannion RJ, et al. Neurotrophins: Peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci USA. 1999;96(16):9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou XF, et al. Differential effects of endogenous brain-derived neurotrophic factor on the survival of axotomized sensory neurons in dorsal root ganglia: A possible role for the p75 neurotrophin receptor. Neuroscience. 2005;132(3):591–603. doi: 10.1016/j.neuroscience.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 87.Eaton MJ, Blits B, Ruitenberg MJ, Verhaagen J, Oudega M. Amelioration of chronic neuropathic pain after partial nerve injury by adeno-associated viral (AAV) vector-mediated over-expression of BDNF in the rat spinal cord. Gene Ther. 2002;9(20):1387–1395. doi: 10.1038/sj.gt.3301814. [DOI] [PubMed] [Google Scholar]
- 88.Miki K, et al. Differential effect of brain-derived neurotrophic factor on high-threshold mechanosensitivity in a rat neuropathic pain model. Neurosci Lett. 2000;278(1–2):85–88. doi: 10.1016/s0304-3940(99)00908-8. [DOI] [PubMed] [Google Scholar]
- 89.Malcangio M, Lessmann V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and trkB receptors. Trends Pharmacol Sci. 2003;24(3):116–121. doi: 10.1016/S0165-6147(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 90.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 91.Castren E. Neurotrophins and psychiatric disorders. Handbook Exp Pharmacol. 2014;220:461–479. doi: 10.1007/978-3-642-45106-5_17. [DOI] [PubMed] [Google Scholar]
- 92.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handbook Exp Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- 93.Hempstead BL. Brain-derived neurotrophic factor: Three ligands, many actions. Trans Am Clin Climatol Assoc. 2015;126:9–19. [PMC free article] [PubMed] [Google Scholar]
- 94.Qiu LL, et al. NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav Immun. 2016;51:109–118. doi: 10.1016/j.bbi.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 95.Tian XS, et al. Surgical stress induces brain-derived neurotrophic factor reduction and postoperative cognitive dysfunction via glucocorticoid receptor phosphorylation in aged mice. CNS Neurosci Ther. 2015;21(5):398–409. doi: 10.1111/cns.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fidalgo AR, et al. Peripheral orthopaedic surgery down-regulates hippocampal brain-derived neurotrophic factor and impairs remote memory in mouse. Neuroscience. 2011;190:194–199. doi: 10.1016/j.neuroscience.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 97.Tajerian M, et al. Brain neuroplastic changes accompany anxiety and memory deficits in a model of complex regional pain syndrome. Anesthesiology. 2014;121(4):852–865. doi: 10.1097/ALN.0000000000000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Monteggia LM, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA. 2004;101(29):10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121(2):341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- 100.Papaleo F, et al. Working memory deficits, increased anxiety-like traits, and seizure susceptibility in BDNF overexpressing mice. Learn Mem. 2011;18(8):534–544. doi: 10.1101/lm.2213711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kolbeck R, Bartke I, Eberle W, Barde YA. Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J Neurochem. 1999;72(5):1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- 102.Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178(2):255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- 103.Sarhan M, et al. BDNF parabrachio-amygdaloid pathway in morphine-induced analgesia. Int J Neuropsychopharmacol. 2013;16(7):1649–1660. doi: 10.1017/S146114571200168X. [DOI] [PubMed] [Google Scholar]
- 104.McGinty JF, Henriksen SJ, Goldstein A, Terenius L, Bloom FE. Dynorphin is contained within hippocampal mossy fibers: Immunochemical alterations after kainic acid administration and colchicine-induced neurotoxicity. Proc Natl Acad Sci USA. 1983;80(2):589–593. doi: 10.1073/pnas.80.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gall C, Berry LM, Hodgson LA. Cholecystokinin in the mouse hippocampus: Localization in the mossy fiber and dentate commissural systems. Exp Brain Res. 1986;62(2):431–437. doi: 10.1007/BF00238862. [DOI] [PubMed] [Google Scholar]
- 106.Marksteiner J, Ortler M, Bellmann R, Sperk G. Neuropeptide Y biosynthesis is markedly induced in mossy fibers during temporal lobe epilepsy of the rat. Neurosci Lett. 1990;112(2–3):143–148. doi: 10.1016/0304-3940(90)90193-d. [DOI] [PubMed] [Google Scholar]
- 107.Gall C, Lauterborn J, Isackson P, White J. Seizures, neuropeptide regulation, and mRNA expression in the hippocampus. Prog Brain Res. 1990;83:371–390. doi: 10.1016/s0079-6123(08)61263-7. [DOI] [PubMed] [Google Scholar]
- 108.Thoenen H, Mueller RA, Axelrod J. Trans-synaptic induction of adrenal tyrosine hydroxylase. J Pharmacol Exp Ther. 1969;169(2):249–254. [PubMed] [Google Scholar]
- 109.Schalling M, Stieg PE, Lindquist C, Goldstein M, Hokfelt T. Rapid increase in enzyme and peptide mRNA in sympathetic ganglia after electrical stimulation in humans. Proc Natl Acad Sci USA. 1989;86(11):4302–4305. doi: 10.1073/pnas.86.11.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Terrando N, et al. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 2013;27(9):3564–3571. doi: 10.1096/fj.13-230276. [DOI] [PubMed] [Google Scholar]
- 111.Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21(17):6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scharfman H, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 113.Dong M, Wu Y, Fan Y, Xu M, Zhang J. c-fos modulates brain-derived neurotrophic factor mRNA expression in mouse hippocampal CA3 and dentate gyrus neurons. Neurosci Lett. 2006;400(1–2):177–180. doi: 10.1016/j.neulet.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 114.Joo JY, Schaukowitch K, Farbiak L, Kilaru G, Kim TK. Stimulus-specific combinatorial functionality of neuronal c-fos enhancers. Nat Neurosci. 2016;19(1):75–83. doi: 10.1038/nn.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J, et al. c-fos regulates neuronal excitability and survival. Nat Genet. 2002;30(4):416–420. doi: 10.1038/ng859. [DOI] [PubMed] [Google Scholar]
- 116.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J Neurosci. 2009;29(41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hovens IB, et al. Postoperative cognitive dysfunction and microglial activation in associated brain regions in old rats. Neurobiol Learn Mem. 2015;118:74–79. doi: 10.1016/j.nlm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 118.Fan D, Li J, Zheng B, Hua L, Zuo Z. Enriched environment attenuates surgery-induced impairment of learning, memory, and neurogenesis possibly by preserving BDNF expression. Mol Neurobiol. 2016;53(1):344–354. doi: 10.1007/s12035-014-9013-1. [DOI] [PubMed] [Google Scholar]
- 119.Kodama D, Ono H, Tanabe M. Increased hippocampal glycine uptake and cognitive dysfunction after peripheral nerve injury. Pain. 2011;152(4):809–817. doi: 10.1016/j.pain.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 120.Waddell G, Pilowsky I, Bond MR. Clinical assessment and interpretation of abnormal illness behaviour in low back pain. Pain. 1989;39(1):41–53. doi: 10.1016/0304-3959(89)90174-7. [DOI] [PubMed] [Google Scholar]
- 121.Attal N, et al. Does cognitive functioning predict chronic pain? Results from a prospective surgical cohort. Brain. 2014;137(Pt 3):904–917. doi: 10.1093/brain/awt354. [DOI] [PubMed] [Google Scholar]
- 122.Berger M, et al. Postoperative cognitive dysfunction: Minding the gaps in our knowledge of a common postoperative complication in the elderly. Anesthesiol Clin. 2015;33(3):517–550. doi: 10.1016/j.anclin.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Avidan MS, Evers AS. The fallacy of persistent postoperative cognitive decline. Anesthesiology. 2016;124(2):255–258. doi: 10.1097/ALN.0000000000000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dokkedal U, Hansen TG, Rasmussen LS, Mengel-From J, Christensen K. Cognitive functioning after surgery in middle-aged and elderly Danish twins. Anesthesiology. 2016;124(2):312–321. doi: 10.1097/ALN.0000000000000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mapplebeck JC, Beggs S, Salter MW. Sex differences in pain: A tale of two immune cells. Pain. 2016;157(Suppl 1):S2–S6. doi: 10.1097/j.pain.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 126.Sorge RE, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nijs J, et al. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: A new therapeutic target? Expert Opin Ther Targets. 2015;19(4):565–576. doi: 10.1517/14728222.2014.994506. [DOI] [PubMed] [Google Scholar]
- 128.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 129.Wall PD, et al. Autotomy following peripheral nerve lesions: Experimental anaesthesia dolorosa. Pain. 1979;7(2):103–111. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- 130.Zhang MD, et al. Neuronal calcium-binding proteins 1/2 localize to dorsal root ganglia and excitatory spinal neurons and are regulated by nerve injury. Proc Natl Acad Sci USA. 2014;111(12):E1149–E1158. doi: 10.1073/pnas.1402318111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi TJ, et al. Phospholipase C{beta}3 in mouse and human dorsal root ganglia and spinal cord is a possible target for treatment of neuropathic pain. Proc Natl Acad Sci USA. 2008;105(50):20004–20008. doi: 10.1073/pnas.0810899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shi TJ, et al. Secretagogin is expressed in sensory CGRP neurons and in spinal cord of mouse and complements other calcium-binding proteins, with a note on rat and human. Mol Pain. 2012;8:80. doi: 10.1186/1744-8069-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Le Maitre E, Barde SS, Palkovits M, Diaz-Heijtz R, Hokfelt TG. Distinct features of neurotransmitter systems in the human brain with focus on the galanin system in locus coeruleus and dorsal raphe. Proc Natl Acad Sci USA. 2013;110(6):E536–E545. doi: 10.1073/pnas.1221378110. [DOI] [PMC free article] [PubMed] [Google Scholar]