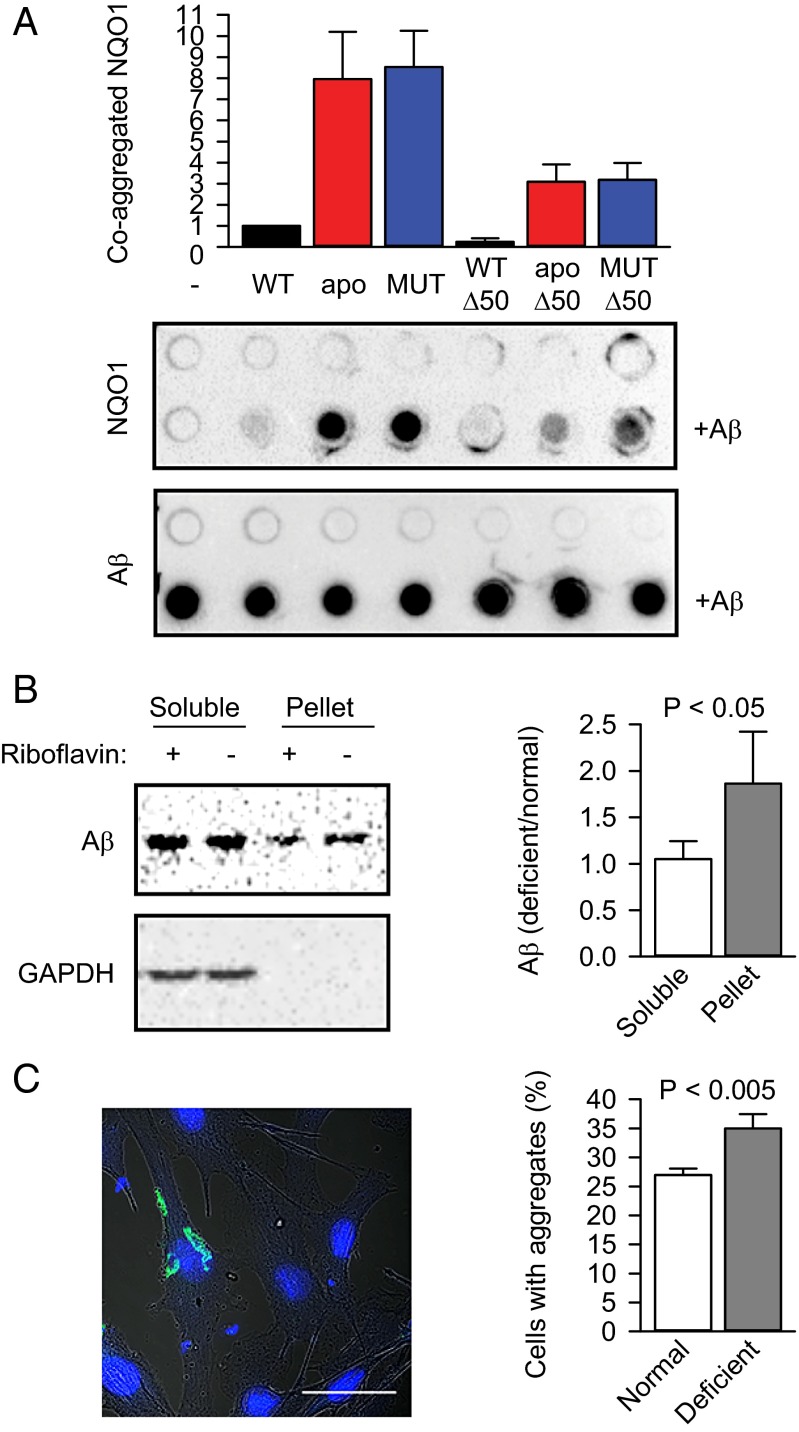
Fig. 5.
Cofactor depletion exacerbates protein aggregation and amyloidogenesis. (A) Coaggregation of NQO1 and its different variants with Aβ1–42 in vitro (n = 3, mean ± SD). apo, apoprotein; apo-Δ50, C-terminally truncated apoprotein; MUT, P187S mutant; MUT-Δ50, C-terminally truncated P187S mutant; WT-Δ50, C-terminally truncated wild-type NQO1. The amount of wild-type NQO1 that coaggregated with the amyloid (+Aβ) was set as 1. Proteins were detected using anti-NQO1 antibody (NQO1) or anti-amyloid antibody (Aβ). (B) The aggregation of transiently transfected Aβ-EGFP (Aβ) in B16 cells was measured using a sedimentation assay. The ratios of Aβ-EGFP in different fractions were normalized to the ratio of Aβ-EGFP in the lysate before ultracentrifugation and are plotted (n = 3, mean ± SD). The significance of the difference between the means was determined using a one-tailed t test and is indicated. (C) The aggregation of transiently transfected Aβ-EGFP in B16 cells was quantified microscopically. One typical cell with aggregated Aβ-EGFP is shown. (Scale bar: 50 μm.) The fractions of cells containing aggregates under normal and riboflavin-deficient conditions are plotted (n = 3, mean ± SD). The significance of the difference between the means was determined using a one-tailed t test and is indicated.