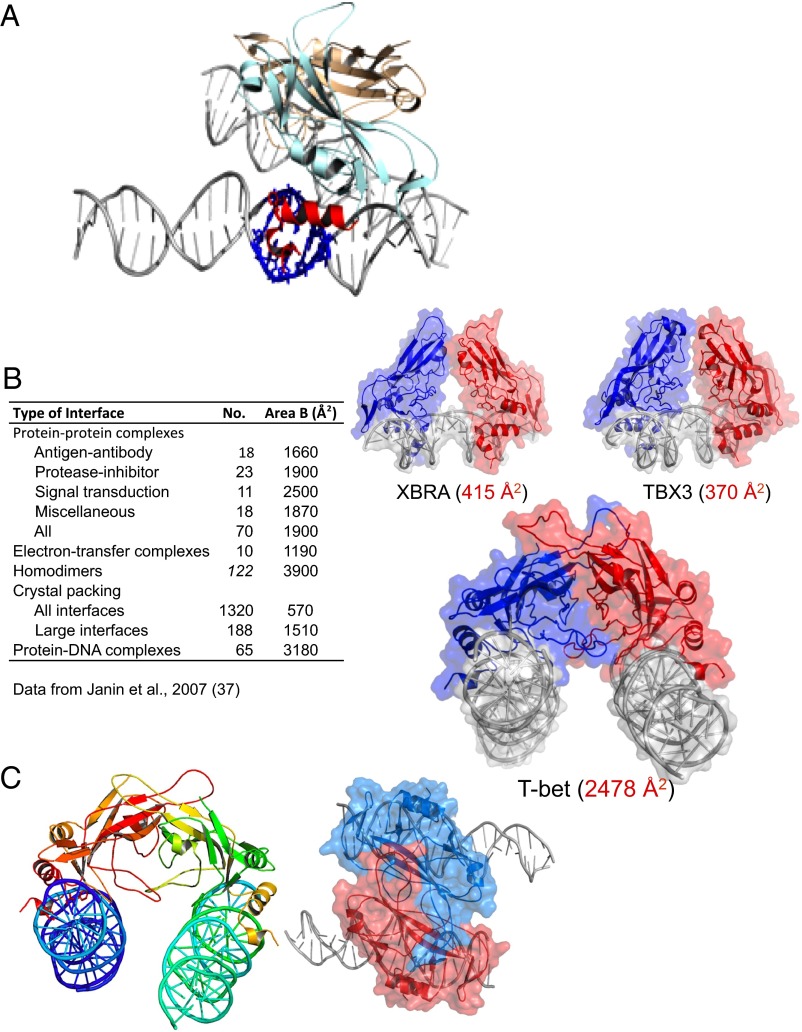
Fig. 2.
The DNA binding domain of T-bet forms a dimer that cross-links two independent DNA strands. (A) The two T-bet DBD monomers (light blue and brown) form a tight dimer. Each monomer binds a single T-bet recognition site on a separate strand of DNA. The protruding helices that make contact with the minor groove of the DNA are shown in red for the light blue monomer; the residues on the double helix that interact with this part of the Tbox domain are shown in navy blue. (B) The T-bet DBD–DNA complex with the molecular surface overlaid, and a similar surface model of the Tbox DBD–DNA complexes of Xbra and TBX3. The Xbra dimer interface is much smaller, and TBX3 binds essentially as two monomers. (C) Orthogonal views of the T-bet Tbox–DNA complex showing binding of two independent DNA strands to each subunit of the dimer. The view on the right has a surface representation overlaid, indicating the tightness of the complex.