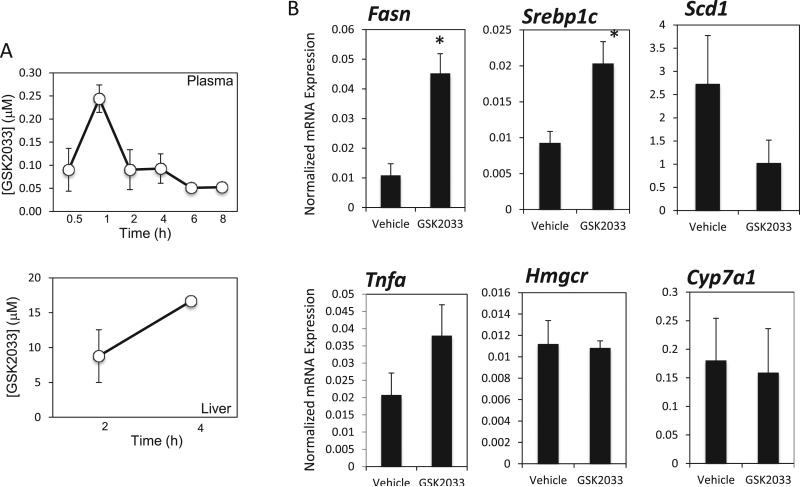
Fig. 2.
Pharmacological action of GSK2033 in vivo. (A) Pharmacokinetic data illustrating that GSK2033 is sustained in plasma over an 8-h time course following a single i. p. injection (30 mg kg). GSK2033 levels remain well above the IC50 in the liver 4-h post i. p. injection (30 mg/kg). (B) GSK2033 displays an unusual pharmacological profile in a mouse model of NAFLD. Following a once daily treatment with 30 mg/kg for 1 month, lipogenic enzyme and proinflammatory gene expression were assessed by qPCR. As illustrated above, expression of Fasn and SREBF1, lipogenic enzymes, appears to be significantly increased following the treatment, indicating that GSK2033 may have mixed agonist/antagonist properties in vivo. The proinflammatory cytokine TNFα displays a trend towards an increase in liver expression, but it is not significant after a 1-month treatment. Unlike SR9238, GSK2033 did not affect the expression of Hmgcr or Cyp7A1 in this study. N = 7, *, indicates p < 0.05 using a Student's t-test.