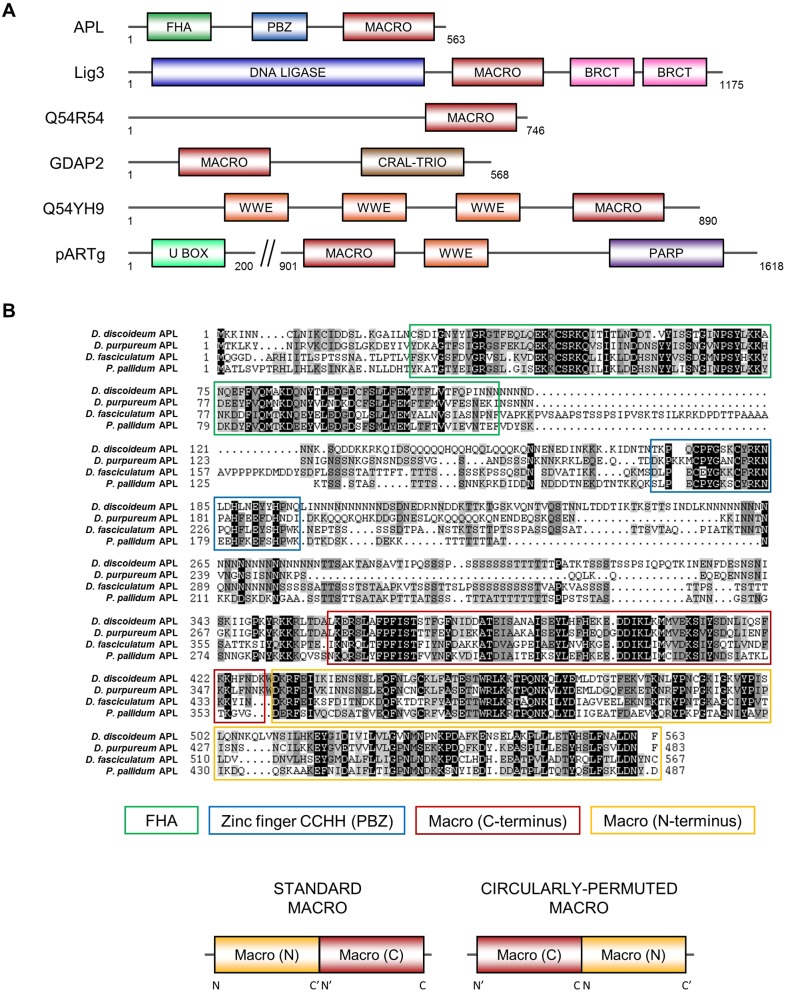
Fig. 1.
The Dictyostelium protein APL contains a circularly permuted macrodomain. (A) Domain structures of the Dictyostelium macrodomain (MACRO)-containing proteins identified through in silico searches. The macrodomains in GDAP2, Q54YH9 (UniProt ID) and pARTg were previously annotated. Domain abbreviations: FHA, forkhead-associated; PBZ, zinc finger CCHH-type; BRCT, BRCA1 C-terminus; CRAL-TRIO, CRAL-TRIO lipid binding domain; U-BOX, U-box domain; PARP, PARP catalytic domain. (B) Multiple sequence alignment of APL from different dictyostelids, highlighting the domain conservation between the proteins. This alignment shows the conservation of a circularly permutated macrodomain, which is illustrated relative to the standard macrodomain. Circular permutation is likely to have arisen from the duplication of the C- and N-terminal regions of successive macrodomains. For this to occur, macrodomains would need to occur in tandem in the progenitor protein, as indeed they do in many extant macrodomain-containing proteins. In the circularly permutated macrodomain, the N- and C-termini of the standard macrodomain lie in the middle of the domain sequence.