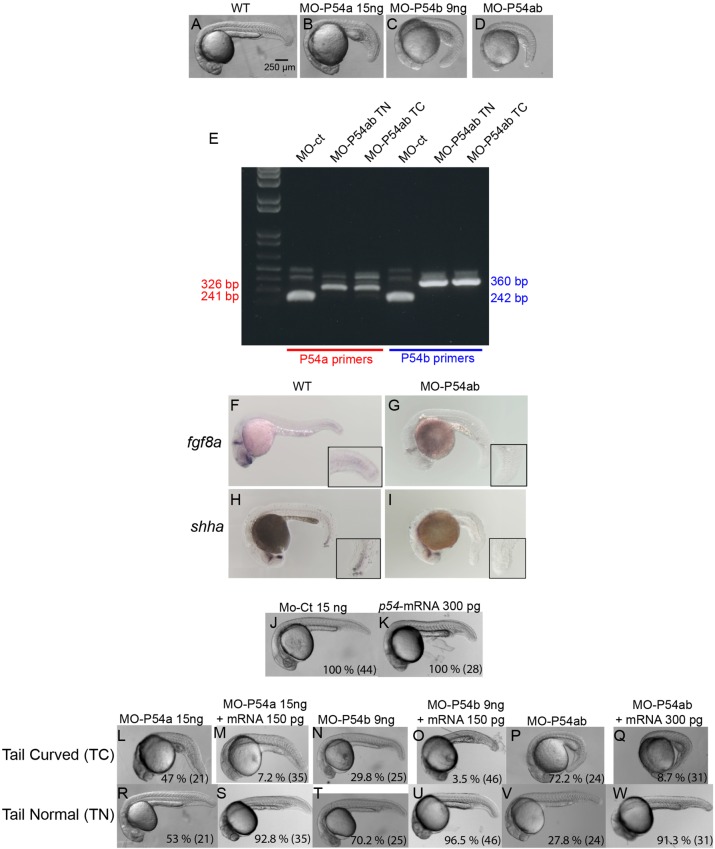
Fig. 5.
Knockdown of P54a and/or P54b RNA helicases in zebrafish embryos. (A–D) show the defects caused by MO-P54a, MO-P54b or MO-P54ab, which mostly affected posterior trunk structures and produce a bend at the end of the trunk (‘tail curved’). (E) Morpholino efficacy was validated by reverse transcription-PCR (RT-PCR). Using specific primers for p54a or p54b mRNA in samples from embryos micro-injected with MO-ct or MO-P54ab. Primers for amplifying p54a and p54b, under normal conditions, produced bands of 241 and 242 bp, respectively. However, in samples from MO-P54b embryos, bands of 326 or 360 bp were observed due to an intron insertion. (F–I) ISH showing the expression of fgf8a and shha in WT and MO-P54ab 24 hpf embryos. (J,K) There were no obvious defects in embryos micro-injected with 15 ng of MO-ct or 300 pg of p54-mRNA. (L–Q) The ‘tail curved’ phenotype produced by MO-P54a, MO-P54b or MO-P54ab was rescued if the morpholino was co-injected with p54-mRNA. (R–W) With every micro-injection, a proportion of embryos developed normally (‘tail normal’), but this proportion increased drastically upon rescue with p54-mRNA. (J–W) For each image, the percentage of embryos showing that phenotype is indicated, with the total number of embryos analyzed shown in parentheses. Control morpholino (MO-ct), p54a morpholino (MO-P54a), p54b morpholino (MO-P54b), mix of p54a and p54b morpholinos (MO-P54ab), ‘in vitro’ synthesized mRNA from p54a (p54-mRNA). ‘in situ’ hybridization (ISH), tail curved phenotype (TC), tail normal (TN).