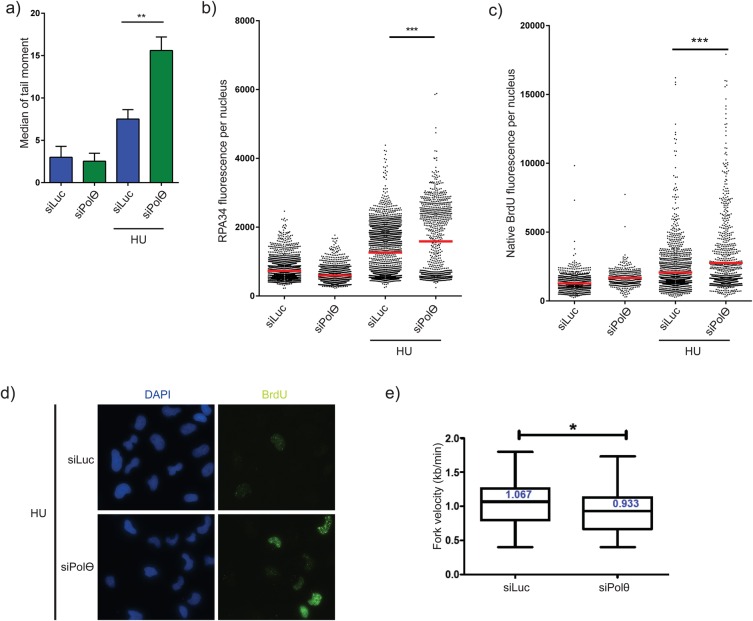
Fig. 4.
Polθ prevents genomic instability induced by replicative stress. (A) Replication-induced DNA DSBs in Polθ-depleted cells after treatment with hydroxyurea (HU). RKO cells were treated with 4 mM of hydroxyurea prior to resuspension in low-melting agarose and electrophoresis required to perform the neutral comet assay. At least 100 nuclei were quantified per condition. A two-tailed t-test was used to assess statistical significance, **P<0.01. The graph shows the mean±s.d. from three independent experiments. (B,C) Increased ssDNA in nuclei from Polθ depleted cells. (B) RKO were treated with indicated doses of hydroxyurea 48 h after transfection cells with control siRNA (siLuc) or siRNAs targeting Polθ. RPA34 intensity was detected in nuclei after nuclear pre-extraction (a minimum of 900 nuclei per condition were quantified). (C) Transfected MRC5-SV were cultivated during 36 h with BrdU in culture medium before treatment with HU. BrdU was detected by immunofluorescence microscopy (a minimum of 350 nuclei per condition were quantified). Two tailed Mann–Whitney tests were performed to assess statistical relevance of populations (***P<0.001). Results of two independent experiments are shown. (D) Representative images of the cells analysed in B,C. (E) Polθ depletion influences fork velocity. Twenty-four hours after transfection of the RKO cell line with control (siLuc) or Polθ siRNA, RKO cells were labelled successively with 50 µM IdU (Sigma-Aldrich) for 15 min, 100 µM CldU for 15 min and 3 h 200 mM thymidine. DNA combing was performed as described previously (Fernandez-Vidal et al., 2014). The number of bi-colour forks analysed in this experiment are 74 and 64 for siLuc and siPOLQ conditions, respectively.