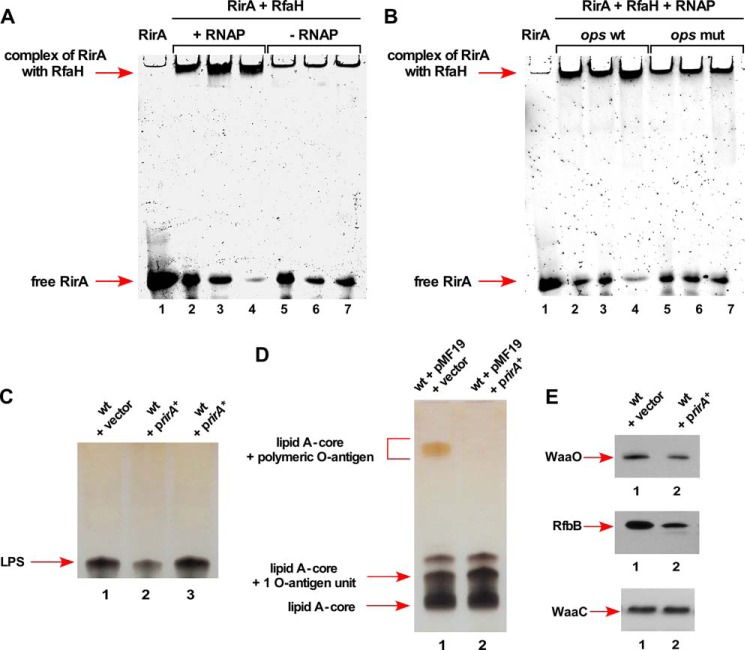
FIGURE 12.
RirA sRNA binds to RfaH and its overexpression causes defects in the LPS synthesis. A, interaction of RirA with RfaH in the presence or absence of RNAP. Fifty ng of RirA were incubated at 37 °C with 150, 300, and 450 ng of RfaH in the presence of 50 μg of RNA polymerase core (lanes 2–4) or in the absence of RNAP (lanes 5–7). Lane 1 corresponds to RirA alone. Samples were analyzed on a 6% native polyacrylamide gel. Arrows indicate the position of complex and free RirA. B, the replacement of the 8-nt ops site by 8 A nt in RirA abolishes interaction with RfaH + RNAP core. Fifty ng of wild-type RirA (lanes 2–4) and RirA with mutated ops site (lanes 5–7) were analyzed for the complex formation with RfaH in the presence of RNAP and resolved by native gel electrophoresis. C, a portion of whole cell lysate obtained from the wild-type E. coli K-12 strain BW25113 with vector pRS551 (lane 1), its derivative expressing the wild-type rirA gene in pRS551 (lane 2), and rirA with 8-nt ops site replaced by 8 A residues rirA* (lane 3) were applied on a 16.5% SDS-Tricine gel and LPS was revealed after silver staining. The arrow indicates the position of the LPS core. D, a portion of whole cell lysates after proteinase K treatment obtained from isogenic strains with pMF19 and vector pRS551 alone (lane 1) and its derivative expressing the rirA gene from its own promoter in pRS551 with pMF19 (lane 2) were resolved on a 14% SDS-Tricine gel and LPS was revealed after silver staining. Arrows indicate the position of the lipid A-core, lipid A-core + 1 O-antigen unit, and lipid A-core + polymeric O-antigen. E, isogenic cultures carrying vector pRS551 alone (lane 1) or expressing the wild-type rirA gene (lane 2) in the strain with chromosomal waaO, rfbB, or waaC genes epitoped at the C-terminal end with 3× FLAG were grown in LB medium at 30 °C up to an A600 of 0.2. An equivalent amount of total protein was analyzed on 12% SDS-PAGE, followed by immunoblotting using anti-FLAG monoclonal antibody.