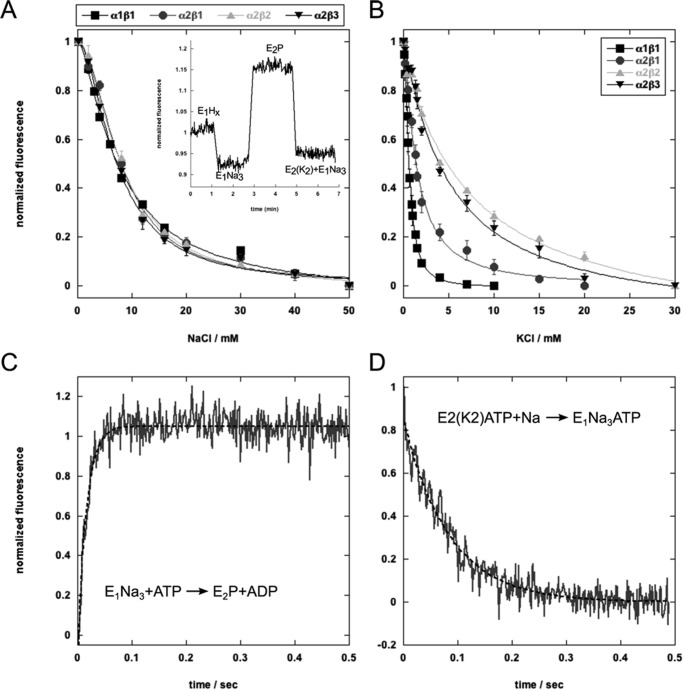
FIGURE 5.
Sodium and potassium binding and conformational changes of the isoforms measured with RH421. A, equilibrium titration of sodium binding to the E1 conformation. The inset shows a standard experiment and fluorescence changes associated with ion binding and release to α2β2 (sodium binding to E1, sodium release and conformational transition to E2P, and potassium binding). B, equilibrium titration of potassium binding to E2P. C, stopped-flow trace of the E1Na3 → E2P transition of α2β2 fitted to a double exponential function. The average of 15 traces is shown. D, stopped-flow trace of the α2β2 E2(Rb2)ATP → E1Na3ATP transition fitted to a single exponential function.