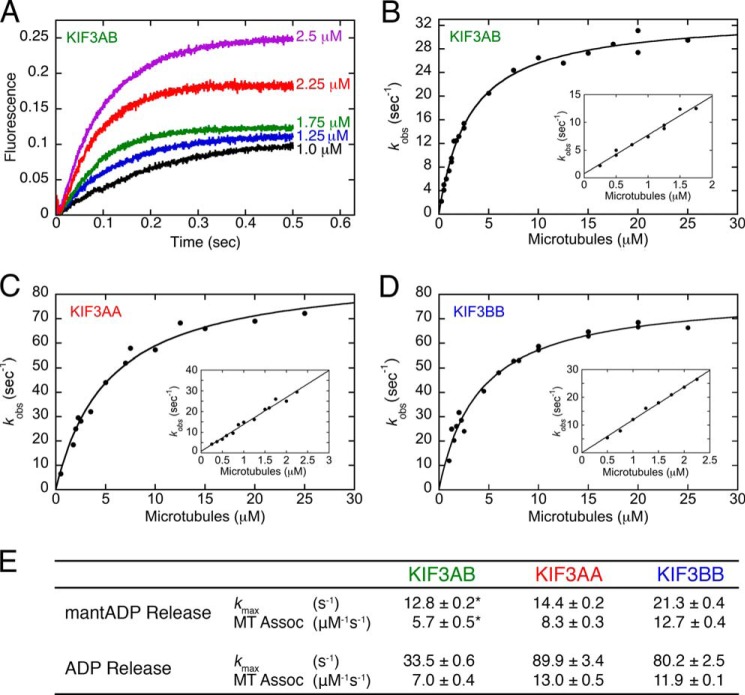
FIGURE 3.
Presteady-state kinetics of MT association followed by ADP release. KIF3 was rapidly mixed in the stopped-flow instrument with varying concentrations of MTs plus mantATP. Final concentrations were 0.25 or 0.5 μm KIF3 sites/25 μm mantATP for 0.25–2.5 μm MTs or 2.5 μm KIF3 sites and 50 μm mantATP for 2.5–25 μm MTs. A, representative KIF3AB transients ranging from 1.0 to 2.5 μm MTs show a biphasic increase in mantATP fluorescence as a function of time. B, the observed rates of each initial fast phase were plotted as a function of MT concentration. The hyperbolic fit provided the maximum rate constant of ADP release at k+2 = 33.5 ± 0.6 s−1 with a K1/2,MTs = 3.1 ± 0.2 μm for KIF3AB. Inset, the initial linear part at low MT concentrations provides the rate constant for MT association: k+1 = 7.0 ± 0.4 μm−1 s−1 and k−1 = 0.8 ± 0.4 s−1. C, KIF3AA maximum rate constant of ADP release at k+2 = 89.9 ± 3.4 s−1 with a K1/2,MTs = 5.3 ± 0.5 μm. Inset, KIF3AA k+1 = 13.0 ± 0.5 μm−1 s−1. D, KIF3BB maximum rate constant of ADP release k+2 = 80.2 ± 2.5 s−1 and K1/2,MTs = 4.0 ± 0.4 μm. Inset, KIF3BB k+1 = 11.9 ± 0.1 μm−1 s−1. E, table summarizing the results. *, the mantADP release kinetics for KIF3AB were reported previously (42) and are from Fig. 2 for KIF3AA and KIF3BB. Assoc, association.