Abstract
Inter-alpha inhibitor proteins (IAIPs) found in relatively high concentrations in human plasma are important in inflammation. IAIPs attenuate brain damage in young and adult subjects, decrease during sepsis and necrotizing enterocolitis in premature infants, and attenuate sepsis-related inflammation in newborn rats. Although a few studies have reported adult organ-specific IAIP expression, information is not available on age-dependent IAIP expression. Given evidence suggesting IAIPs attenuate brain damage in young and adult subjects, and inflammation in newborns, we examined IAIP expression in plasma, cerebral cortex (CC), choroid plexus (CP), cerebral spinal fluid (CSF), and somatic organs in fetal, newborn, and adult sheep to determine the endogenous expression patterns of these proteins during development. IAIPs (enzyme-linked immunosorbent assay) were higher in newborn and adult than fetal plasma (P<0.05). Western immunoblot detected 125 kDa PaI (Pre-alpha Inhibitor) and 250 kDa IaI (Inter-alpha Inhibitor) in plasma, CNS, and somatic organs. PaI expression in CC and CP was higher in fetuses than newborns and adults, but IaI expression was higher in adults than fetuses and newborns. Both PaI and IaI were higher in fetal than newborn CSF. IAIPs exhibited organ-specific ontogenic patterns in placenta, liver, heart, and kidney. These results provide evidence for the first time that plasma, brain, placenta, liver, heart, and kidney express IAIPs throughout ovine development and that expression patterns are unique to each organ. Although exact functions of IAIPs in CNS and somatic tissues are not known, their presence in relatively high amounts during development suggests their potential importance in brain and organ development.
Keywords: Brain development, cerebral spinal fluid, inter-alpha inhibitor proteins, ontogeny, sheep
Introduction
Inter-alpha inhibitor proteins (IAIPs) are a family of structurally related proteins found in mammalian plasma in relatively high concentrations. IAIPs play an important role in inflammation as part of innate immunity, wound healing, and cancer metastasis.1–3 Major forms found in human plasma are Inter-alpha Inhibitor (IaI), which consists of two heavy chains (H1 and H2) and a single light chain, and Pre-alpha Inhibitor (PaI), which consists of one heavy (H3) and one light chain. High levels of circulating IAIPs normally found in plasma of adults and newborns, and even in prematurely born infants, suggest that these proteins are important. Moreover, complete absence of IAIPs has not been reported in humans, suggesting that these proteins have important functions in human biology.1 In premature infants, IAIPs recently have been shown to decrease in association with sepsis and necrotizing enterocolitis.4–6 In addition, both disorders are associated with increased incidences of brain damage in premature infants.7,8
Although the physiological functions of IAIPs remain to be established, current findings suggest that these molecules are part of innate immunity and play a critical role during inflammation. IAIPs have unique immunomodulatory roles during sepsis in neonatal rats.2,9,10 Bikunin or urinary trypsin inhibitor also has been suggested to be effective in inhibiting premature delivery most likely by suppressing cytokines and other inflammatory mediators.11–15 Bikunin knockout mice have increased sensitivity to lipopolysaccharide-induced death in vivo.16,17 Bikunin carries a chondroitin sulfate chain to which the heavy chains are covalently linked.11 The heavy chains can be transferred from IAIPs to hyaluronan molecules and become covalently linked in the extracellular matrix.11,18 Heavy chains linked to hyaluronan molecules have also been found in inflamed tissues.18 However, the physiological role of heavy chains of IAIPs is not known. Taken together, these results suggest that proteins of the IAIP family, including bikunin, are anti-inflammatory agents. These functions potentially explain its beneficial effects in systemic inflammation and suggest that IAIPs could play an important role in inflammation-related disorders during the perinatal period.
IAIP related molecules and mRNA have been detected in neurons, astrocytes, and meningeal cells of the brain.19–29 Recent work suggests associations between variations in phenotypes of the heavy chain alleles with brain disorders such as schizophrenia and bipolar disease30 suggesting that IAIPs might be important to normal brain development.
Although there is very little information regarding the effects of these molecules on the brain, bikunin may have neuroprotective properties against the development of stroke-related ischemic injury in adult rats,31 protect oligodendrocytes from apoptosis, and promote remyelination in an experimental autoimmune encephalitis model in adult rats.32 The light chain of IAIPs, also called bikunin or urinary trypsin inhibitor or ulinastatin, has recently been shown to have neuroprotective properties in young piglets exposed to hypothermic low-flow cardiopulmonary bypass potentially via its anti-inflammatory properties.33 Bikunin has also been shown to block the production of inflammatory cytokines during reperfusion after ischemic injury in several somatic organs.34–38 Nonetheless, there is very little information regarding the expression of these molecules in brain and other organs during development.20,39
The choroid plexus (CP) produces cerebral spinal fluid (CSF), which provides physical protection for the brain, and removes brain metabolites via drainage of the CSF. However, studies that are more recent suggest that the CP-CSF system also plays a much more active role in development, homeostasis, and repair of the central nervous system (CNS).40–42 The CP is a highly specialized tissue, strategically positioned within the ventricles to provide the CNS with variety of biologically active growth factors essential for normal brain development.41–43 These factors include neurotrophic and angiogenic factors, which are potentially involved in neurogenesis and axonal guidance during CNS development, and in responses to brain injury and during the subsequent repair processes.44–53 Previous studies reported that many species during development, including human premature infants, have very high CSF protein concentrations that are most likely important for brain development.54–57 Therefore, proteins found in CSF most likely influence brain development and responses to injury. Very limited information suggests that urinary trypsin inhibitor is present in CSF of adults, particularly in those with brain tumors and in the postoperative state.58 However, levels of IAIPs have not been previously examined in the CSF of any species during development.
Information is also very limited regarding the organ-specific distribution of these critical molecules, including in brain. In humans, IAIPs were detected in cerebrum, cerebellum, lungs, kidney, liver, colon, skin, and testes.21 Information is not available regarding the expression of IAIPs in the brain or somatic organs during normal development in any species.
The ovine fetus has been widely used to investigate brain development.59–61 The neurodevelopment of the immature ovine brain is similar to that of the premature infant with respect to completion of neurogenesis, onset of cerebral sulcation, and detection of the cortical component of the auditory evoked potentials.59,62,63 Full term in sheep pregnancy is 148 days of gestation. The preterm fetal sheep brain between 94 and 96 days of gestation is comparable to that of the preterm infant between 24 and 28 weeks of gestation, whereas fetal sheep at 135 days of gestation is similar to that of the near-term human infant.64 In the present study, we obtained samples of brain and somatic organs over a wide range of ages to examine changes in IAIPs expression over a broad developmental window. Although rodents are frequently used to study brain development, the rodent brain is immature at birth64 and almost completely agyric. In contrast, similar to the nonhuman primate and human brain, the sheep brain develops prenatally and is gyrencephalic.
In summary, IAIPs attenuate inflammatory responses during the perinatal period,9,11–15 decrease during sepsis and necrotizing enterocolitis in premature infants,5,6 and are important in ischemic and inflammatory related brain and somatic organ damage.31,32 Given the potential importance of these molecules in perinatal period inflammation, and in brain and somatic organ damage, as an initial approach to understand these critical molecules during development, we examined levels of IAIPs in the plasma, brain, CP, CSF, and somatic organs throughout ovine development with a primary focus on developmental expression patterns of IAIPs in brain.
Materials and methods
The present study was conducted after approval by the Institutional Animal Care and Use Committees of Brown University and Women & Infants Hospital of Rhode Island and according to the National Institutes of Health Guidelines for use of experimental animals.
Animal preparation and experimental design
Plasma, cerebral cortical, CP, CSF, placenta, liver, heart, and kidney tissues samples for the present study were frozen samples obtained from placebo-treated sham-operated control animals from previous studies.61,65–67 Samples from all age groups were obtained over similar time intervals. Surgical procedures and physiological measures were performed for the former studies.61,65–68 As described previously in detail,61,65–67 surgery was performed under ketamine (10 mg/kg) and 1–2% halothane anesthesia in pregnant ewes at 60% (87–90 days), 70% (106–107 days), 90% (135–138 days) of gestation; newborn lambs (4–6 days of age); and adult nonpregnant sheep (3 years of age). The sham-operated control animals from our previous studies were sacrificed without further intervention. Plasma samples were obtained from all animals just before the euthanasia. At the end of the studies, a CSF sample was obtained from the fetal and newborn sheep via a direct puncture of the allantoic membrane. The sample was inspected for blood contamination and discarded if there was evidence of contamination. CSF samples were not available from the adult sheep. Tissues, plasma, and CSF were snap frozen in liquid nitrogen and remained at −80°C until analysis. The number of animals per group for different organs available for analysis is summarized in Table 1. Although CP samples were not available from fetuses at 70% gestation, samples were available from fetal sheep at 60% gestation.
Table 1.
Number of plasma, cerebral cortex, CP, CSF, placental, liver, heart, and kidney samples by age group
| Groups | Plasma | Cerebral cortex | Choroid plexus | CSF | Placenta | Liver | Heart | Kidney |
|---|---|---|---|---|---|---|---|---|
| 60% (87–90 days of gestation) | – | – | 5 | – | – | – | – | – |
| 70% (106–107 days of gestation) |
5 | 8 | – | 4 | 5 | 6 | 6 | 6 |
| 90% (135–138 days of gestation) |
7 | 8 | 5 | 4 | 5 | 6 | 5 | 6 |
| Newborn (4–6 days) | 4 | 8 | 5 | 5 | – | 6 | 5 | 5 |
| Adult (3 years) | 3 | 3 | 3 | – | – | 3 | 3 | 3 |
(–) indicates sample not available.
Competitive enzyme-linked immunosorbent assay (ELISA) to measure IAIPs level in ovine plasma and CSF
IAIPs concentrations were measured by a competitive ELISA in sheep plasma using a polyclonal antibody raised against human IAIPs (R-20 pAb). The polyclonal antibody was generated by immunizing rabbits with highly purified human plasma derived IAIPs.69 The R-20 pAb cross-reacts with nonhuman IAIPs including sheep and detects both 250 kDa IaI and 125 kDa PaI molecules in Western immunoblot analysis. Furthermore, this R-20 pAb binds to heavy chains as well as light chain of human IAIPs after enzymatic digestion.69 Ninety-six-well high-binding microplates Microlon 600 (Greiner Bio-One, Monroe, NC, USA) were coated with purified sheep IAIPs. Sheep IAIPs were purified from sheep serum (Quad Five, Ryegate, MO, USA) by anion-exchange chromatography on a Toyopearl Q-600 C-AR column (Tosoh Bioscience, King of Prussia, PA, USA). Bound IAIPs were eluted with a buffer containing 750 mM NaCl. The purified sheep IAIPs were diluted in 100 mM NaPO4 buffer pH 6.5 and immobilized on the microplates (50 ng/per well) for 1 h at room temperature or overnight at 4°C. Subsequently, the microplate was blocked with 200 µL of 5% nonfat dried milk in phosphate buffered saline (PBS) and 0.05% Tween. Sheep plasma was diluted in PBS and a known amount of purified sheep IAIPs was serially diluted in PBS to establish a standard curve for quantitative analysis of IAIP concentrations in the samples. After 50 µL of samples and serially diluted IAIPs standards were added to the wells, 50 µL of R-20 PAb diluted in 1:1200 in PBS was added to each well. Plates were incubated for 1 h at room temperature and subsequently washed with PBS and 0.05% Tween using automated plate washer (Biotek EL-404, Winooski, VT, USA). The bound R-20 pAb was detected by adding HRP-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. After washing, 100 -µL Enhanced K-Blue TMB substrate (Neogen Corp, Lexington, KY, USA) was added to the wells and the reaction was stopped by adding 100 µL 1 N HCl solution. The absorbance at 450 nm was measured on SpectraMAX Plus microplate reader (Molecular Devices, Sunnyvale, CA, USA). Each sample was tested in triplicate and assays were repeated at least twice on all samples.
Preparation of cytosolic tissue fractions
Cell cytosolic fractions of cerebral cortex (CC), CP, placenta, liver, heart, and kidney for IAIPs were extracted in buffer A (TRIS 10 mM pH 6.8, Sucrose, MgCl) with 1% complete protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Total protein concentrations of the homogenates were determined with a bicinchoninic acid protein assay (BCA, Pierce, Rockford, IL, USA). Aliquots of the extracted samples were stored at −80°C. We examined the cytosolic fraction for this study because the primary antibody R-20 recognized IAIPs only in the cytosol.
Ovine IAIP
To investigate the relationship between IaI (250 kDa) and PaI (125 kDa) in adult sheep plasma, ovine IAIPs were purified from ovine serum by anion-exchange chromatography. Sheep serum (Quad Five, Ryegate, MT) was obtained and filtered to remove large precipitates and bacteria. A preparative chromatographic system (BioCad, Applied Biosystems) was used for two successive separation steps: a quaternary ion exchange (QA-R) column (Tosoh Bioscience, King of Prussia, PA) and a monolithic CIMmultus DEAE anion exchange column (BIA Separations, Villach, Austria). The wash method involved buffers of varying pH and salt concentrations in order to remove other contaminating serum proteins, thereby obtaining highly pure IAIP.
Western immunoblot detection and quantification of proteins
A total of 15–50 µg protein of total protein per well (CC: 50 µg, CP: 15 µg, CSF: 22.5 µL, plasma: 1 µL from 1:100 dilution; placenta: 30 µg, liver: 50 µg, heart: 50 µg, and kidney: 50 µg) were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and polyvinyl diflouride membrane (SDS-PAGE) and transferred onto polyvinyl diflouride (PVDF) membranes (0.2 µm, Bio-Rad Laboratories, Hercules, CA) using a semi-dry technique. Membranes were each incubated with a rabbit polyclonal antibody against human IAIPs (R-20 pAb, ProThera Biologics, East Providence, RI, USA) at a dilution of 1:5000. The immunoblots were incubated in primary antibody overnight at 4°C. Peroxidase-labeled secondary antibody goat anti-rabbit (Alpha Diagnostic, San Antonio, TX, USA) was incubated for 1 h at room temperature in a dilution of 1:10,000. Binding of the secondary antibody was detected with enhanced chemiluminescence (ECL plus, Western Blotting Detection reagents, Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA) before exposure to autoradiography film (Daigger, Vernon Hills, IL, USA).
Experimental samples were normalized to a reference protein standard that was obtained from a homogenate protein pool from the tissues of a single adult sheep. For the purpose of this report, we refer to these samples as internal control (IC) samples. As we have previously described,70–73 these samples served as an IC for quality of loading, transfer of the samples, normalization of the densitometric values, and to permit accurate comparisons among the different immunoblots.70,71,74 The use of IC is unique to our laboratory and allows us to compare large groups of animals over a large number of different immunoblots. We developed this methodology because investigation of a large number of housekeeping proteins showed that they all exhibited significant variations during ovine development mitigating their use as housekeeping proteins. The experimental protein autoradiographic densitometrical values were expressed as a ratio to the IC, thus facilitating normalized comparisons among different groups and multiple immunoblots. When this methodology was used within a single age group (newborn), the method correlates well with values that were normalized as ratios to β-actin.70
Each immunoblot included samples from the four groups and three IC samples. The IC samples were included in three lanes, as the first, middle, and last samples on each immunoblot. We calculated a coefficient of variation for the IC samples on each immunoblot. The values for the experimental samples were accepted as valid only if the percent coefficient of variation for the IC samples was less than 20% on the immunoblot. Human plasma-derived IAIPs served as a positive control (PC) for all immunoblots to ascertain that the antibody correctly identified the ovine proteins. Molecular weight standards (Bio-Rad Laboratories, Hercules, CA, USA) were included in each immunoblot. The rabbit polyclonal anti-IAIP (R-20 pAb) detected IAIPs bands at 125 and 250 kDa (PaI and IaI) in all organs. Uniformity in inter-lane loading was also established by Coomassie blue (Sigma, St. Louis, MO, USA) staining of the polyacrylamide gels and uniformity of transfer to the polyvinylidene difluoride membranes was confirmed by Ponceau S staining (Sigma, St. Louis, MO, USA).
Samples from all animals and age groups were placed on each immunoblot to permit identical standard conditions among all of the tissue samples from the different age groups. However, in order to facilitate graphic clarity, to clarify the age group comparisons for each tissue, and to place the immunoblots above the bar graphs on the same figures, we selected the one specific immunoblot that most closely represented the mean expression value for each specific age group for the bar graph value of each tissue. Illustrations displaying the entire gels in the figures would have not adequately emphasized differences among the various age groups with respect to the IAIP expression.
Densitometric analysis
Band intensities were analyzed with a Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD, USA). All experimental samples were normalized to the respective average of the three IC samples. However, the band intensities were expressed as integrated optical density (IOD) units for CP and CSF as we did not have adult CP or CSF. The final values represented averages of the densitometry values obtained from the different immunoblots (plasma, n = 2; CC, n = 8; CP, n = 5; CSF, n = 2; placenta, n = 5; liver, n = 5; heart, n = 5; kidney, n = 5) and were presented as a ratio to the IC sample except for CP and CSF.
Statistical analysis
All results were expressed as standard error of the mean (± SEM). Two-way analysis of variance (ANOVA) was used to compare the differences among the groups. The factors were age group (fetuses at 60%/70%, 90% of gestation, newborn, and adult) and protein expression (125 kDa PaI and 250 kDa IaI). When a significant difference was detected by ANOVA, the Fisher’s least significant difference test was used to further describe the statistically significant differences among the groups. P<0.05 was considered statistically significant.
Results
IAIPs detection by SDS-PAGE, ELISA, and Western immunoblot in ovine plasma
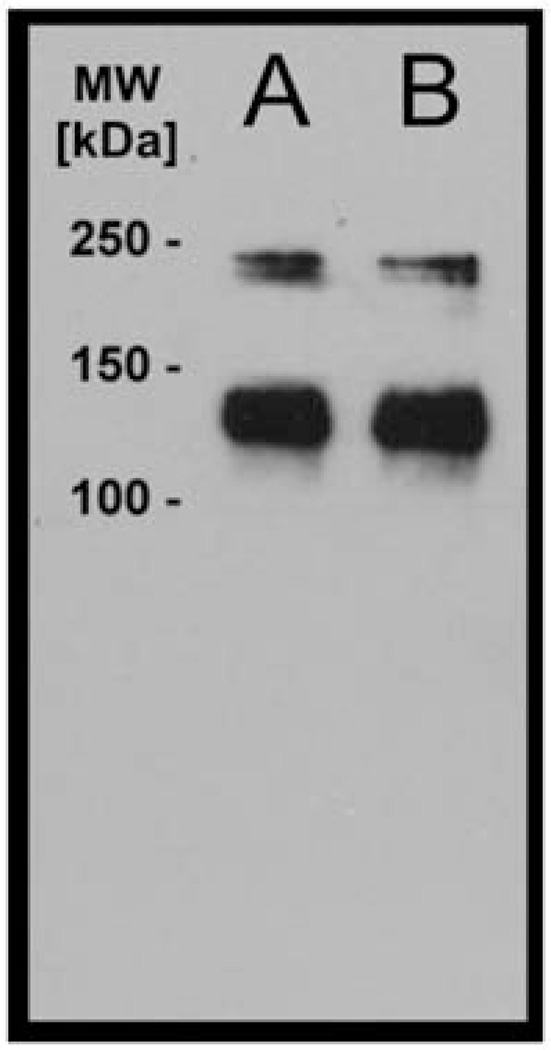
The SDS-PAGE and Western immunoblot illustrate that ovine IAIPs contain two major proteins, IaI and PaI with the purity of 85–90% (Figure 1). The Western immunoblot (Figure 2) comparing adult human (Figure 2(a)) with adult ovine plasma (Figure 2(b)) illustrates that both human and ovine plasma contain the IaI (250 kDa) and the PaI (125 kDa) with similar apparent molecular weights.
Figure 1.
SDS-PAGE illustrating purification of ovine IAIPs and Western immunoblot of the purified ovine IAIPs illustrate that ovine IAIPs consist of two major proteins, the IaI (250 kDa) and the PaI (125 kDa). IaI: Inter alpha Inhibitor; PaI: Pre alpha Inhibitor.
Figure 2.
Western immunoblot comparing adult human (a) with adult ovine plasma (b) shows that both human and ovine plasma contain the IAIPs (IaI and PaI) protein moieties at similar apparent molecular weights (MW)
IAIPs detected by competitive sheep ELISA in ovine plasma were lower (P<0.05) in the fetuses at 70 and 90% gestation than in the newborn lambs and lower in the fetuses at 90% of gestation than in adult sheep (Figure 3). The IAIPs were detected as 125 kDa PaI and 250 kDa IaI bands in ovine plasma by Western immunoblot (Figure 4). The expression of 125 kDa PaI did not differ among the age groups. In contrast, the expression of 250 kDa IaI was lower in the fetuses at 70 and 90% gestation and in the newborn lambs than in the adult sheep.
Figure 3.
Plasma concentrations of IAIPs plotted for the different age groups. Fetuses at 70 and 90% gestation have significantly lower plasma IAIP concentrations than newborn lambs, *P < 0.05 versus newborn lambs, and fetuses at 90% gestation have significantly lower plasma IAIP concentrations than the adult sheep, +P < 0.05 versus adult sheep. Values are mean ± SEM. IAIP: inter-alpha inhibitor protein
Figure 4.
125 kDa PaI and 250 kDa IaI expression in ovine plasma plotted as the ratio to the IC standard for the different age groups. (a) 125 kDa PaI: Expressions did not differ among the different age groups. (b) 250 kDa IaI: Fetuses at 70 and 90% and newborn lambs have significantly lower expression than the adult sheep. *P < 0.05 versus adult sheep. IC designates the band for IC standard. Values are mean ± SEM
IAIPs detection by Western immunoblot in CC, CP, and CSF
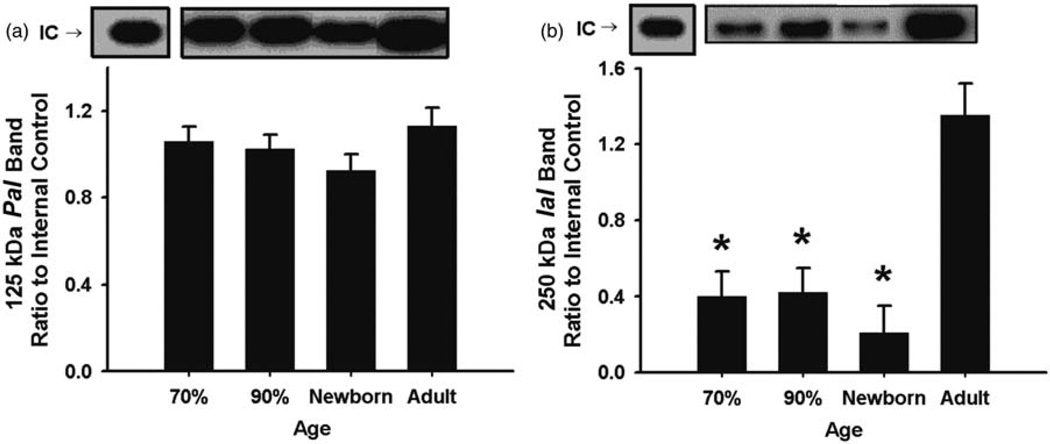
IAIPs were detected in CC, CP, and CSF as 125 kDa PaI and 250 kDa IaI protein bands by Western immunoblot using the specific antibody against IAIPs (Figures 5 to 8). The expression of PaI was higher in the CC in fetuses at 70% of gestation than in the newborn lambs and higher in the adult sheep than in the newborn lambs (Figure 5(a)). The 250 kDa IaI expression was lower in the CC in the fetuses at 90% gestation and in the newborn lambs than in the adult sheep (Figure 5(b)).
Figure 5.
125 kDa PaI and 250 kDa IaI expression in ovine cerebral cortex plotted as the ratio to the IC standard for the different age groups. (a) 125 kDa PaI: Expression was higher in fetuses at 70% of gestation and in the adult sheep than in the newborn lambs. *P < 0.05 versus newborn lambs. (b) 250 kDa IaI: Expression was lower in fetuses at 90% of gestation and in newborn lambs than in the adult sheep. *P < 0.05 versus adult sheep. IC designates the band for IC standard. Values are mean ± SEM
Figure 8.
125 kDa PaI and 250 kDa IaI expression plotted as arbitrary IOD units in CSF for the fetuses at 70 and 90% of gestation and newborn lambs. (a) 125 kDa PaI and (b) 250 kDa IaI: Expressions were higher in fetuses at 70 and 90% gestation than in newborn lambs. *P < 0.05 versus newborn lambs. Values are mean ± SEM
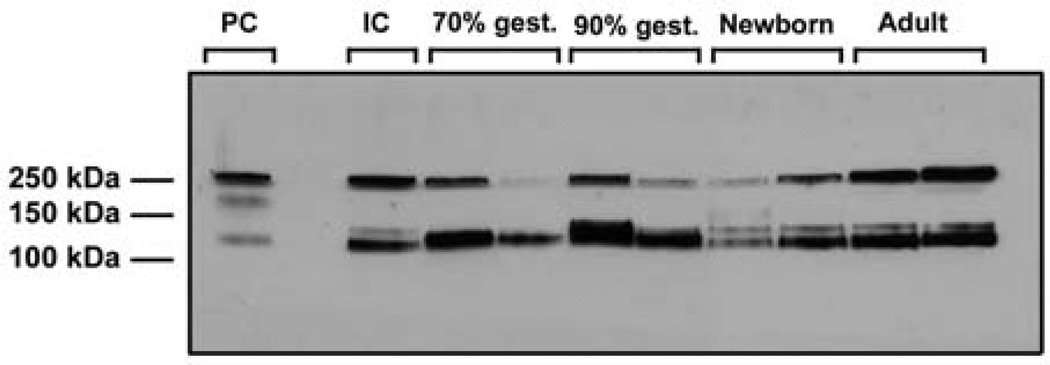
Figure 6 shows a representative Western immunoblot for cerebral cortical IAIP expression in order to illustrate the appearance of original immunoblots. The immunoblot contains two representative animals for each age group. The expression of the 125 kDa PaI and 250 kDa IaI proteins is shown for the ovine cerebral cortical samples in the fetuses at 70 and 90% of gestation and in the newborn and adult sheep. Two types of standards were used on all immunoblots. The human plasma-derived IAIPs were used as PCs in all immunoblots to be certain that we identified the correct IAIP bands in each ovine plasma, tissue, and CSF sample. As described previously,70–73,75 the IC protein standard was also used on each immunoblot; this sample protein had been obtained from the single adult sheep brain and was used for normalization for all of the tissues and age groups.
Figure 6.
125 kDa PaI and 250 kDa IaI expression in ovine cerebral cortex at 70 and 90% of gestation, newborn, and adult. Human plasma-derived Inter-alpha Inhibitor proteins used as a PC. IC standard protein derived from the adult cerebral cortex as described in the “Materials and methods” section. IC: internal control; PC: positive control
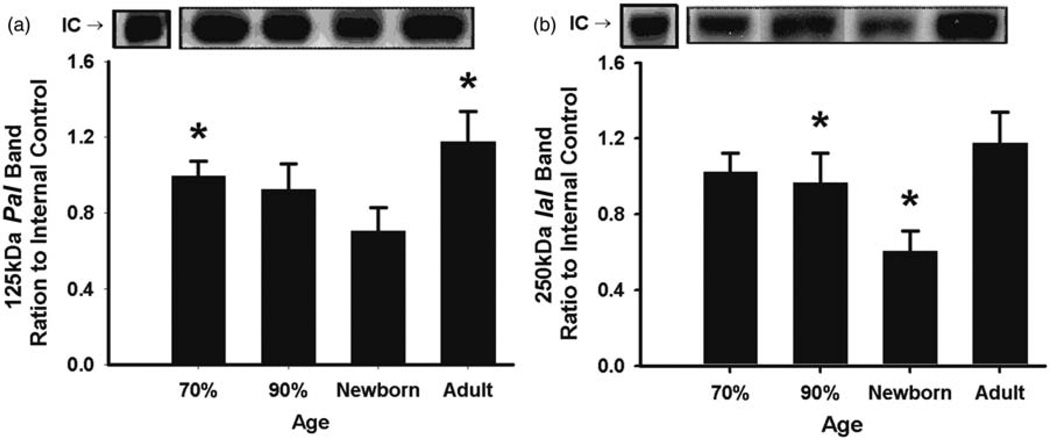
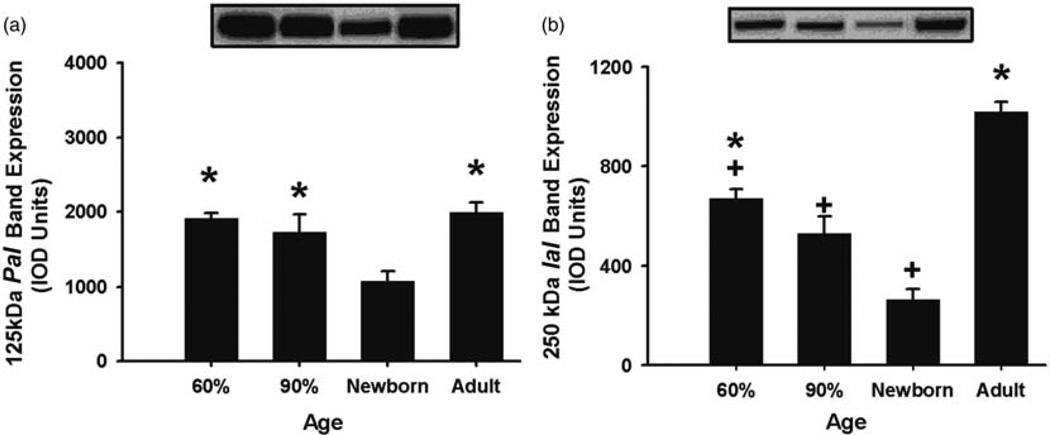
The expression of the PaI in CP was lower in newborn lambs than in the fetuses at 60 and 90% of gestation and in the adult sheep (Figure 7(a)), and IaI expression was higher in the fetuses at 60% of gestation and in the adult sheep than in the newborn lambs, but lower in the fetuses at 60 and 90% gestation than in the adult sheep (Figure 7(b)). The expression of the PaI and IaI in CSF was higher in fetuses at 70 and 90% of gestation than in the newborn lambs (Figure 8(a) and (b)). The IAIPs levels in the CSF were below the limit of detection by the sheep-specific ELISA. In summary, the CC, CP, and CSF, each exhibit distinct patterns of expression for the 125 kDa PaI and 250 kDa IaI. However, both molecules appear lower in the newborn lambs than in the fetuses at 70 and 90% of gestation in CP and CSF. We do not know the pattern of expression in adult sheep, as we did not have samples from the adult sheep.
Figure 7.
125 kDa PaI and 250 kDa IaI expression plotted as arbitrary integrated optical density (IOD) units in the choroid plexus for the different age groups. (a) 125 kDa PaI: Expression is higher in the fetuses at 60 and 90% gestation and in the adult sheep than in the newborn lambs, *P < 0.05 versus newborn lambs. (b) 250 kDa IaI: Expression band was higher in fetuses at 60% gestation and in the adult sheep than in the newborn lambs and lower in fetuses at 60 and 90% gestation and newborn lambs than in the adult sheep. *P < 0.05 versus newborn lambs, +P < 0.05 versus adult sheep. Values are mean ± SEM
Table 2 contains the expression of the 125 kDa PaI and 250 kDa IaI in the different somatic organs by age group as ratio to the IC proteins. IAIPs were also detected as PaI and IaI in placenta, liver, heart, and kidney in fetal, newborn, and adult sheep. In placenta, the PaI and IaI expressions did not differ significantly between the fetuses at 70 and 90% of gestation. In the liver, PaI was higher in fetuses at 70 and 90% gestation than the newborn lambs and lower in the newborn lambs than in the adult sheep. IaI expression was lower in the fetuses at 70 and 90% of gestation and in the newborn lambs than in the adult sheep and higher in the fetuses at 90% of gestation than in the newborn lambs. In the heart, the expression of PaI did not differ among the groups. In contrast, IaI was higher in the fetuses at 70 and 90% of gestation than in the newborn lambs. In the kidney, the PaI band expression was lower in fetuses at 90% gestation than in the fetuses at 70% of gestation and in the adult sheep, but the expression of IaI was lower in fetuses at 70 and 90% of gestation and in the newborn lambs than in the adult sheep.
Table 2.
IAIP values in somatic organs by age group as ratio to the internal control protein standard
| Organ | Age | |||
|---|---|---|---|---|
| 70% | 90% | Newborn | Adult | |
| Placenta | ||||
| 125 kDa PaI | 0.73 ± 0.21 | 1.51 ± 0.21 | – | – |
| 250 kDa IaI | 1.27 ± 0.43 | 1.28 ± 0.43 | – | – |
| Liver | ||||
| 125 kDa PaI | 0.99 ± 0.11* | 0.92 ± 0.11* | 0.71 ± 0.11 | 1.18 ± 0.18 |
| 250 kDa IaI | 0.29 ± 0.07† | 0.47 ± 0.07† | 0.14 ± 0.07† | 0.77 ± 0.10 |
| Heart | ||||
| 125 kDa PaI | 1.64 ± 0.10 | 1.30 ± 0.09 | 1.07 ± 0.11 | 1.55 ± 0.13 |
| 250 kDa IaI | 3.83 ± 0.91* | 3.68 ± 0.83* | 1.67 ± 1.01 | 2.10 ± 1.17 |
| Kidney | ||||
| 125 kDa PaI | 1.36 ± 0.10 | 0.87 ± 0.10†‡ | 1.16 ± 0.10 | 1.37 ± 0.15 |
| 250 kDa IaI | 0.53 ± 0.13† | 0.38 ± 0.13† | 0.34 ± 0.13† | 1.1 ± 0.18 |
IaI: Inter alpha Inhibitor; PaI: Pre alpha Inhibitor.
Values are mean ± SEM, expressed as ratio to internal control. (–) value not available.
P < 0.05 versus newborn,
P < 0.05 versus adult,
P < 0.05 versus fetuses at 70% of gestation.
Inspection of Figures 5, 7, and 8 compared with Figure 4 suggests that the pattern of expression of IAIPs in the brain, CP, and CSF differed from those in the plasma for the same age groups. We had brain vascular volume measurements available from our previous experiments determined by using intravenous [3H]-sucrose (MW 344) in the fetuses at 70% gestation76 and [3H]-polyethylene glycol (MW 1000) in the fetuses at 90% gestation, newborn, and adult sheep.61,77 Based upon these vascular volume measurements, we were able to calculate the estimated mean IAIP concentrations contained within the brain vasculature per gram brain tissue (Table 3). Inspection of Table 3 suggests that the amount of IAIPs contained within the brain vasculature represented a relatively small amount of the total IAIPs contained within the brain parenchyma.
Table 3.
Estimated mean inter-alpha inhibitor proteins concentration per gram brain tissue (µg/g)
| 70% Gestation | 90% Gestation | Newborn | Adults | |
|---|---|---|---|---|
| Mean blood volume (µL/g) | 41 | 35 | 22 | 17 |
| Mean plasma IAIP conc. (µg/mL) | 64 | 55 | 111 | 102 |
| Estimated mean IAIP conc. (µg/g) | 2.6 | 1.9 | 2.4 | 1.7 |
Table 4 schematically summarizes the 125 kDa PaI and 250 kDa IaI expression for each organ in the fetuses at 60 or 70% and 90% of gestation, newborn lambs compared with the adult sheep.
Table 4.
Schematic summary of 125 kDa PaI and 250 kDa IaI protein expression compared with adult values for each tissue by age
| 125 kDa PaI expression | 250 kDa IaI expression | |||||
|---|---|---|---|---|---|---|
| Organ | Age 60%/70% |
90% | Newborn | Age 60%/70% |
90% | Newborn |
| Plasma | ↔ | ↔ | ↔ | ↓ | ↓ | ↓ |
| Brain | ↔ | ↔ | ↓ | ↔ | ↓ | ↓ |
| CP | ↔ | ↔ | ↓ | ↓ | ↓ | ↓ |
| Liver | ↔ | ↔ | ↔ | ↓ | ↓ | ↓ |
| Heart | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Kidney | ↔ | ↓ | ↔ | ↓ | ↓ | ↓ |
↑ indicates significant increase compared to adult, ↓ indicates significant decrease compared to adult, ↔ indicates no change compared with adult values. CSF not listed because adult samples were not available.
Discussion
The main purpose of our study was to examine the expression of IAIPs in the brain and to compare changes in brain with those in plasma and somatic organs of sheep during development as an initial approach to understanding these critical molecules during development. The presence of IAIPs was identified for the first time in plasma, CC, CP, liver, heart, and kidney from early in fetal life and through the neonatal period up to maturity in adult sheep, and in the placenta and CSF during fetal life as both the 125 kDa PaI and 250 kDa IaI. The findings of our study are novel because to the best of our knowledge previous work has not reported distributions of IAIPs in the brain and somatic organs during a wide span of development in any species. The major findings of this study were as follows. (1) The level of IAIPs increases in plasma after birth. (2) The 125 kDa PaI expression was higher in the adult and fetal than in newborn lamb cerebral cortices, but the 250 kDa IaI expression was higher in adult than fetal and newborn cerebral cortices. (3) The expression of PaI and IaI in CP was highest in the adult sheep. (4) PaI and IaI were high in CSF of fetal sheep and very low in newborn lambs after birth. (5) IAIPs exhibit ontogenic patterns of expression specific to each molecular species and organ. The presence of both molecules of IAIPs with organ-specific patterns of expression during ovine development may be interpreted to suggest that these proteins have important immunomodulatory1,2 functions during organ development.
Recent studies have shown relatively high levels of circulating IAIPs are normally present in adult human plasma2,78 and even in plasma of premature infants.4–6 Our findings during ovine development extend these observations in human plasma and suggest that the concentrations of IAIPs, measured by ELISA, increase markedly after birth. In addition, the expression of the IAIP-related molecules, which contribute to the total amount of IAIPs measured by ELISA, differs with respect to their expression during development such that the expression of the 125 kDa PaI is similar at all ages, but that of the 250 kDa IaI increases markedly after birth. This finding suggests that it is the 250 kDa IaI moiety that contributes to the high levels of total IAIPs observed in adult sheep plasma. However, although the level of the total IAIPs (measured by ELISA) is high in the newborn lambs, the expression of the 250 kDa IaI appears low in the lambs, suggesting that 125 kDa PaI moiety is more likely to contribute to the relatively high levels of the total IAIP protein after birth.
IAIPs and related proteins have previously been localized in various tissues in adult rodents and humans, including cerebrum and cerebellum, lung, liver, intestines, colon, kidney, bladder, testes, and skin.21,79–81 IAIPs also have been shown to exhibit a specific distribution within the brains of mice and rats with localization primarily in CC, hippocampus, and hypothalamus.82 Unfortunately, we only had residual cerebral cortical and CP samples from our previous studies,61,65–67 so that we cannot comment on the amounts of IAIPs expressed in other brain regions. However, in the CC, we observed different ontogenic patterns for the 125 kDa PaI and 250 kDa IaI, such that the former was higher in the fetuses at 70% of gestation and in adult sheep than in newborns, but the later higher in the adult sheep than in the newborns and fetuses at 90% of gestation. Although we cannot comment upon the distribution of IAIPs in other brain regions, identify the localization of IAIPs to specific cell types, or identify the biological functions of IAIPs from our study, others have reported that IAIPs are most likely produced within the neurons and/or astrocytes in the murine brain, because intense immunoreactivities were localized to neuronal processes.28,82
Inflammation plays a key role in many CNS disorders.83 There is now evidence to suggest that bidirectional communications between the CNS and periphery could contribute to acute and chronic CNS disorders.83 Increased levels of IAIPs in ovine plasma and CNS tissue during development could be related to the importance of these molecules in systemic and CNS inflammatory and immunological responses.1,2 Recent evidence suggests that bikunin, the light chain of IAIPs, reduces oxidative stress, early inflammation, and endothelial activation in the forebrain of rats84; reduces ischemia–reperfusion-related delayed neuronal apoptosis in gerbils85; protects against white matter demyelination and oligodendrocytes from apoptosis; and promotes remyelination in a model of experimental autoimmune encephalomyelitis.32 In addition, bikunin attenuates polymorphonuclear neutrophil infiltration and decreases infarct volume in ischemic-reperfusion injury in the brain of adult rats.31 Moreover, endogenous bikunin appears to be directly involved in repair process of injured neurons,31 and protease inhibitors derived from neuronal cells function as regulators of neurite regeneration and outgrowth.86 A recent study also suggests that urinary trypsin inhibitors have neuroprotective properties in young piglets exposed to hypothermic low-flow cardiopulmonary bypass potentially via its anti-inflammatory properties.33 Hence, IAIPs appear to have a variety of important neuroprotective effects in several animal models. Therefore, based upon our findings identifying the presence of IAIPs in relatively large amounts throughout ovine development, we speculate that these molecules could potentially represent endogenous anti-inflammatory molecules with neuroprotective properties and/or be important to brain development.
The patterns of IAIP expression in the CP were somewhat similar to those of the CC during development. CSF is produced as an ultrafiltrate of plasma by the CP and from drainage of interstitial fluid from CNS tissues. Approximately 80% of the total amount of protein in CSF originates from blood with the remaining 20% originating directly from the CNS.87 CSF in adults has much lower protein concentrations than plasma due to restricted entry of blood-derived components through the blood–CSF barrier.41 Most abundant proteins in plasma are also elevated in CSF with exception of proteins forming large complexes, and, consequently, exhibiting very low diffusion rates into CSF.41 High concentrations of protein have been previously reported in the immature CSF of fetal sheep56 and in newborn and preterm infants with levels several times higher than those in adults.88 The higher protein concentrations in fetal CSF are most likely a result of local production by CP rather than immaturity of the blood–brain or blood–CSF barriers because the blood–brain and blood–CSF barriers form very early during development in the fetus.41,55,77,88
The high levels of both the 125 kDa PaI and 250 kDa IaI molecules expressed in CSF in the fetal sheep, which decrease after birth, are consistent with findings of elevated levels of other proteins during gestation in several other species including rodents, pigs, rabbits, chickens, and in premature infants.55,88–92 Although initially it was thought that elevated protein concentrations in CSF simply reflected an immature leaky blood–brain barrier to proteins during development, more recent information suggests that elevated CSF proteins in the fetus and newborn most likely have important roles for brain growth and development.41 The protein composition of CSF in the early stages of fetal development is very complex. The majority of proteins are low molecular weight proteins such as albumin, alpha-fetoprotein, transferrin, lipoproteins, etc. the concentrations of which show significant variations during different stages of development.55,93 These protein fractions most likely represent molecules that have important biological functions including growth factors and cytokines, which could influence the development of neuroepithelial cells.41,89,94
The ontogenic patterns of protein concentration in fetal CSF have been studied in several species. In the chick and sheep, CSF protein concentrations increase during the late fetal period and decrease just before birth.55,95,96 In contrast, a similar decrease does not occur until after birth in rats,54 suggesting that phylogenic differences play a role in the pattern of protein expression in CSF during maturation. A large proportion of brain development in the sheep occurs before birth,97,98 but the majority of the rodent brain growth occurs after birth.97 Differences between patterns of protein concentrations in sheep and rodent CSF most likely reflect maturational differences in brain development among species.97
IAIPs have been previously detected in human CSF in patients with brain tumors and inflammatory diseases, but their levels were not affected by systemic levels.58 Numerous studies suggest that the high concentration of protein in fetal CSF is not due to simple diffusion from plasma, rather there are specific developmentally regulated transfer mechanisms in the CP.43,99–105 Inspection of Figures 3 and 8 suggests that this phenomenon is true in sheep as the IAIP levels in CSF are higher in the fetuses than in newborn lambs, but the plasma IAIPs levels are higher in newborn lambs than in fetuses. Therefore, we speculate that the presence of relatively high levels of IAIPs in fetal CSF is probably due to local synthesis by the CP and brain parenchyma during critical periods of brain development, and that these molecules in CSF could be important in brain growth in the fetus.
In the present study, endogenous IAIPs were detected for the first time during the development in the sheep CNS. Furthermore, they were detected in relatively high amounts in the CC and CP at all stages of development and in the CSF during fetal life. However, expression in CC, CP, and CSF decreased in newborn lambs after delivery. Although we cannot discern from our study, the reason that the levels of IAPs decreased dramatically after birth, we speculate that the stress of delivery along with endogenous hormonal changes could have affected the CSF levels of IAIPs after birth. The levels in CC and CP increased again in adult sheep, most likely related to the importance of these proteins in innate immunity. Even though we cannot be certain of the physiological significance of our findings of high IAIPs levels in ovine brain, CP, and CSF during development, we speculate that these molecules represent important factors for brain development.
Similar to our findings in the brain, we have shown for the first time that these immunomodulatory proteins are present in somatic organs and in the placenta, and that they exhibit distinct molecular weight and organ-specific patterns of developmental regulation in liver, heart, kidney, and placenta. Based upon our findings that IAIPs have unique patterns of expression in brain, CP, CSF, placenta, liver, heart, and kidney, which differ from those observed in sheep plasma, it appears unlikely that the serum IAIP levels could account for the organ-specific patterns of expression. Moreover, the brain vascular IAIP levels do not appear to be major contributors to the patterns of IAIP expression within the brain (Table 3). We speculate that although the exact functions of IAIPs are not known, their presence in large amounts with organ-specific variations during development raises the possibility that they represent endogenous anti-inflammatory molecules with organ-specific differential production or modulation during development.
Although we detected the expression of both IAIP proteins in the placenta, we have not measured the potential maternal to fetal transfer of IAIP proteins across the placenta, which would be of great interest for future studies. It would be feasible to radiolabel IAIP proteins and measure their kinetics across the placenta.106 The type of the placenta could affect protein transfer from the maternal to fetal compartments. Moreover, the transfer of other proteins such as maternal antibodies varies dramatically among species during the gestation.107,108 In carnivores, some mammals, and rodents, the placenta transfer of antibody can occur from mother to fetus.107,108 In contrast, the placenta of ruminants such as bovine and ovine species forms a barrier between maternal and fetal circulations that impedes the transfer of antibodies.109,110 In these species, most of the antibodies are delivered to the newborn via maternal milk. In the human, IAIP levels in newborns do not appear to be dependent upon maternal levels potentially suggesting that fetal levels could be independent of maternal levels.4 However, the relationship between maternal to fetal transfer of IAIP proteins awaits further investigation.
There are several limitations to our study. We did not have CSF samples from adult sheep available and, consequently could not compare adult values with those from the fetuses and newborn lambs. However, IAIPs are not detectable in CSF from healthy adult humans (Y.-P. Lim 2014, un-published personal communications), but are increased in the presence of inflammation and tumors.58 We also did not have samples properly saved from our previous studies to determine the specific immunohistochemical location of IAIPs within the brain and we did not have tissue available from other brain regions. Consequently, we cannot comment upon the cellular localization of IAIPs or on their expression in other brain regions. The identity of the ovine IAIPs in this study is based on the reactivity of R-20 pAb that was raised against human IAIPs and was cross-reactive with ovine IAIPs. This antibody has been shown to react against heavy chains and light chain molecules of human IAIPs.69 Although the composition of the specific molecular moieties of IAIP has not been characterized in sheep, identification of the molecular moieties of ovine IAIPs and their functional characteristics are beyond the scope of the current study. Finally, we only measured protein levels of IAIPs in the brain and somatic tissues, which is also dependent on the protein turnover rate. Although quantitative real-time-polymerase chain reaction (qRT-PCR) could more accurately reflect the IAIP tissue measurements than differences in the protein expression patterns, we did attempt to develop qRT-PCR measures for ovine IAIPs, but ovine IAIPs have not been cloned and, hence, the ovine primers were not available. We attempted to match available bovine IAIPs primers with the ovine genome to create several different potential ovine primers. However, only one of the primers gave us a signal consistent throughout the different tissue types. Unfortunately, this primer yielded a much larger sequence than initially anticipated. Thus, we speculated that the recognized sequence was the ovine IAIPs. Unfortunately, although we attempted TOPO A cloning, the procedure was not successful. We consider it important to pursue this area of research in future work. Nonetheless, it should be emphasized that our work is important, as there are no previous studies addressing the quantitative distribution of IAIPs among different tissue types in a single species over this wide developmental time span.
We conclude that IAIPs exhibit specific patterns of expression in the CNS and somatic organs of sheep during development. Although exact functions of IAIP are not known in CNS and somatic tissues, their presence in relatively high amounts suggests their importance to brain and organ development.
Acknowledgments
This work was supported by [R01-HD-057100] from the National Institutes of Health, National Institutes for Child Health and Human Development (NICHD), and National Institutes for Neurological Diseases and Stroke (NINDS); [RI-INBRE P20RR016457-11] from the National Center for Research Resources (NCRR); [RIRA 2010-42] from the Rhode Island Research Alliance Collaborative Grant Awards, and 2011–2012 Klaus Perinatal Research Award from the American Academy of Pediatrics to MSS.
Footnotes
Author contributions: MSS designed the study, performed the analysis of the tissues, analyzed and interpreted the data, and wrote the initial draft of the manuscript; GBS contributed to the experimental design and assisted with the analysis of the tissues and interpretation of the data; SWT contributed to the experimental design and assisted with the analysis of the tissues and interpretation of the data; Y-PL purified the IAIPs and developed and supervised the ELISA for IAIPs and Western immunoblot analysis, developed and provided the reagents specific to these studies; BSS supervised the study design, all tissue and statistical analysis, interpretation of the data, and revised the manuscript.
REFERENCES
- 1.Fries E, Blom AM. Bikunin — not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000;32:125–137. doi: 10.1016/s1357-2725(99)00125-9. [DOI] [PubMed] [Google Scholar]
- 2.Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, Palardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis. 2003;188:919–926. doi: 10.1086/377642. [DOI] [PubMed] [Google Scholar]
- 3.Salier JP, Rouet P, Raguenez G, Daveau M. The inter-alpha-inhibitor family: From structure to regulation. Biochem J. 1996;315:1–9. doi: 10.1042/bj3150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek YW, Brokat S, Padbury JF, Pinar H, Hixson DC, Lim YP. Inter-alpha inhibitor proteins in infants and decreased levels in neonatal sepsis. J Pediatr. 2003;143:11–15. doi: 10.1016/S0022-3476(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 5.Chaaban H, Shin M, Sirya E, Lim YP, Caplan M, Padbury JF. Inter-alpha inhibitor protein level in neonates predicts necrotizing enterocolitis. J Pediatr. 2010;157:757–761. doi: 10.1016/j.jpeds.2010.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaaban H, Singh K, Huang J, Siryaporn E, Lim YP, Padbury JF. The role of inter-alpha inhibitor proteins in the diagnosis of neonatal sepsis. J Pediatr. 2009;154:620–622. e1. doi: 10.1016/j.jpeds.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 7.O’Shea TM. Cerebral palsy in very preterm infants: New epidemiological insights. Ment Retard Dev Disabil Res Rev. 2002;8:135–145. doi: 10.1002/mrdd.10032. [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 9.Singh K, Zhang LX, Bendelja K, Heath R, Murphy S, Sharma S, Padbury JF, Lim YP. Inter-alpha inhibitor protein administration improves survival from neonatal sepsis in mice. Pediatr Res. 2010;68:242–247. doi: 10.1203/PDR.0b013e3181e9fdf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang TC, Zhang SW, Sun LN, Wang H, Ren AM. Magnolol attenuates sepsis-induced gastrointestinal dysmotility in rats by modulating inflammatory mediators. World J Gastroenterol. 2008;14:7353–7360. doi: 10.3748/wjg.14.7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhuo L, Hascall VC, Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem. 2004;279:38079–38082. doi: 10.1074/jbc.R300039200. [DOI] [PubMed] [Google Scholar]
- 12.Castillo GM, Templeton DM. Subunit structure of bovine ESF (extracellular-matrix stabilizing factor(s)). A chondroitin sulfate proteoglycan with homology to human I alpha i (inter-alpha-trypsin inhibitors) FEBS Lett. 1993;318:292–296. doi: 10.1016/0014-5793(93)80531-x. [DOI] [PubMed] [Google Scholar]
- 13.Sanggaard KW, Hansen L, Scavenius C, Wisniewski HG, Kristensen T, Thogersen IB, Enghild JJ. Evolutionary conservation of heavy chain protein transfer between glycosaminoglycans. Biochim Biophys Acta. 2010;1804:1011–1019. doi: 10.1016/j.bbapap.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Stergiakouli E, Hamshere M, Holmans P, Langley K, Zaharieva I, Hawi Z, Kent L, Gill M, Williams N, Owen MJ, O’Donovan M, Thapar A. Investigating the contribution of common genetic variants to the risk and pathogenesis of ADHD. Am J Psychiatry. 2012;169:186–194. doi: 10.1176/appi.ajp.2011.11040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garantziotis S, Hollingsworth JW, Ghanayem RB, Timberlake S, Zhuo L, Kimata K, Schwartz DA. Inter-alpha-trypsin inhibitor attenuates complement activation and complement-induced lung injury. J Immunol. 2007;179:4187–4192. doi: 10.4049/jimmunol.179.6.4187. [DOI] [PubMed] [Google Scholar]
- 16.Wakahara K, Kobayashi H, Yagyu T, Matsuzaki H, Kondo T, Kurita N, Sekino H, Inagaki K, Suzuki M, Kanayama N, Terao T. Bikunin suppresses lipopolysaccharide-induced lethality through down-regulation of tumor necrosis factor-alpha and interleukin-1 beta in macrophages. J Infect Dis. 2005;191:930–938. doi: 10.1086/428134. [DOI] [PubMed] [Google Scholar]
- 17.Yagyu T, Kobayashi H, Matsuzaki H, Wakahara K, Kondo T, Kurita N, Sekino H, Inagaki K. Enhanced spontaneous metastasis in bikunin-deficient mice. Int J Cancer. 2006;118:2322–2328. doi: 10.1002/ijc.21293. [DOI] [PubMed] [Google Scholar]
- 18.Fries E, Kaczmarczyk A. Inter-alpha-inhibitor, hyaluronan and inflammation. Acta Biochim Pol. 2003;50:735–742. [PubMed] [Google Scholar]
- 19.Daveau M, Jean L, Soury E, Olivier E, Masson S, Lyoumi S, Chan P, Hiron M, Lebreton JP, Husson A, Jegou S, Vaudry H, Salier JP. Hepatic and extra-hepatic transcription of inter-alpha-inhibitor family genes under normal or acute inflammatory conditions in rat. Arch Biochem Biophys. 1998;350:315–323. doi: 10.1006/abbi.1997.0515. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez D, Martinez S, Lindqvist A, Akerstrom B, Falkenberg C. Expression of the AMBP gene transcript and its two protein products, alpha(1)-microglobulin and bikunin, in mouse embryogenesis. Mech Dev. 2002;117:293–298. doi: 10.1016/s0925-4773(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 21.Businaro R, Leali FM, De Renzis G, Pompili E, Pagliari G, Menghi G, Fumagalli L. Inter-alpha-trypsin inhibitor-related immunoreactivity in human tissues and body fluids. Cell Mol Biol. 1992;38:463–471. [PubMed] [Google Scholar]
- 22.Cai T, Yu P, Monga SP, Mishra B, Mishra L. Identification of mouse itih-4 encoding a glycoprotein with two EF-hand motifs from early embryonic liver. Biochim Biophys Acta. 1998;1398:32–37. doi: 10.1016/s0167-4781(98)00049-9. [DOI] [PubMed] [Google Scholar]
- 23.Kashyap RS, Nayak AR, Deshpande PS, Kabra D, Purohit HJ, Taori GM, Daginawala HF. Inter-alpha-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clin Chim Acta. 2009;402:160–163. doi: 10.1016/j.cca.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Kato M, Seki N, Sugano S, Hashimoto K, Masuho Y, Muramatsu M, Kaibuchi K, Nakafuku M. Identification of sonic hedgehog-responsive genes using cDNA microarray. Biochem Biophys Res Commun. 2001;289:472–478. doi: 10.1006/bbrc.2001.5976. [DOI] [PubMed] [Google Scholar]
- 25.Mizushima S, Nii A, Kato K, Uemura A. Gene expression of the two heavy chains and one light chain forming the inter-alpha-trypsin-inhibitor in human tissues. Biol Pharm Bull. 1998;21:167–169. doi: 10.1248/bpb.21.167. [DOI] [PubMed] [Google Scholar]
- 26.Takano M, Mori Y, Shiraki H, Horie M, Okamoto H, Narahara M, Miyake M, Shikimi T. Detection of bikunin mRNA in limited portions of rat brain. Life Sci. 1999;65:757–762. doi: 10.1016/s0024-3205(99)00302-1. [DOI] [PubMed] [Google Scholar]
- 27.Werbowetski-Ogilvie TE, Agar NY, Waldkircher de Oliveira RM, Faury D, Antel JP, Jabado N, Del Maestro RF. Isolation of a natural inhibitor of human malignant glial cell invasion: Inter alpha-trypsin inhibitor heavy chain 2. Cancer Res. 2006;66:1464–1472. doi: 10.1158/0008-5472.CAN-05-1913. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida E, Yoshimura M, Ito Y, Mihara H. Demonstration of an active component of inter-alpha-trypsin inhibitor in the brains of Alzheimer type dementia. Biochem Biophys Res Commun. 1991;174:1015–1021. doi: 10.1016/0006-291x(91)91520-m. [DOI] [PubMed] [Google Scholar]
- 29.Chan P, Risler JL, Raguenez G, Salier JP. The three heavy-chain precursors for the inter-alpha-inhibitor family in mouse: new members of the multicopper oxidase protein group with differential transcription in liver and brain. Biochem J. 1995;306:505–512. doi: 10.1042/bj3060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamshere ML, Walters JT, Smith R, Richards AL, Green E, Grozeva D, Jones I, Forty L, Jones L, Gordon-Smith K, Riley B, O’Neill T, Kendler KS, Sklar P, Purcell S, Kranz J, Morris D, Gill M, Holmans P, Craddock N, Corvin A, Owen MJ, O’Donovan MC. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatry. 2013;18:708–712. doi: 10.1038/mp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003;98:465–473. doi: 10.1097/00000542-200302000-00028. [DOI] [PubMed] [Google Scholar]
- 32.Shu Y, Yang Y, Qiu W, Lu Z, Li Y, Bao J, Feng M, Hu X. Neuroprotection by ulinastatin in experimental autoimmune encephalomyelitis. Neurochem Res. 2011;36:1969–1977. doi: 10.1007/s11064-011-0520-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Xue Q, Yan F, Li L, Liu J, Li S, Hu S. Ulinastatin as a neuroprotective and anti-inflammatory agent in infant piglets model undergoing surgery on hypothermic low-flow cardiopulmonary bypass. Paediatr Anaesth. 2013;23:209–216. doi: 10.1111/pan.12073. [DOI] [PubMed] [Google Scholar]
- 34.El Maradny E, Kanayama N, Halim A, Maehara K, Kobayashi T, Terao T. Effects of urinary trypsin inhibitor on myometrial contraction in term and preterm deliveries. Gynecol Obstet Invest. 1996;41:96–102. doi: 10.1159/000292051. [DOI] [PubMed] [Google Scholar]
- 35.Kakinuma C, Kuwayama C, Kaga N, Futamura Y, Katsuki Y, Shibutani Y. Trophoblastic apoptosis in mice with preterm delivery and its suppression by urinary trypsin inhibitor. Obstet Gynecol. 1997;90:117–124. doi: 10.1016/S0029-7844(97)00176-2. [DOI] [PubMed] [Google Scholar]
- 36.Kaga N, Katsuki Y, Futamura Y, Obata M, Shibutani Y. Role of urinary trypsin inhibitor in the maintenance of pregnancy in mice. Obstet Gynecol. 1996;88:872–882. doi: 10.1016/0029-7844(96)00268-2. [DOI] [PubMed] [Google Scholar]
- 37.Cao ZL, Okazaki Y, Naito K, Ueno T, Natsuaki M, Itoh T. Ulinastatin attenuates reperfusion injury in the isolated blood-perfused rabbit heart. Ann Thorac Surg. 2000;69:1121–1126. doi: 10.1016/s0003-4975(99)01433-2. [DOI] [PubMed] [Google Scholar]
- 38.Nakahama H, Obata K, Sugita M. Ulinastatin ameliorates acute ischemic renal injury in rats. Ren Fail. 1996;18:893–898. doi: 10.3109/08860229609047715. [DOI] [PubMed] [Google Scholar]
- 39.Salier JP, Chan P, Raguenez G, Zwingman T, Erickson RP. Developmentally regulated transcription of the four liver-specific genes for inter-alpha-inhibitor family in mouse. Biochem J. 1993;296:85–91. doi: 10.1042/bj2960085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johanson CE, Stopa EG, McMillan PN. The blood-cerebrospinal fluid barrier: Structure and functional significance. Methods Mol Biol. 2011;686:101–131. doi: 10.1007/978-1-60761-938-3_4. [DOI] [PubMed] [Google Scholar]
- 42.Johansson PA, Dziegielewska KM, Liddelow SA, Saunders NR. The blood-CSF barrier explained: When development is not immaturity. Bioessays. 2008;30:237–248. doi: 10.1002/bies.20718. [DOI] [PubMed] [Google Scholar]
- 43.Johansson PA, Dziegielewska KM, Ek CJ, Habgood MD, Liddelow SA, Potter AM, Stolp HB, Saunders NR. Blood-CSF barrier function in the rat embryo. Eur J Neurosci. 2006;24:65–76. doi: 10.1111/j.1460-9568.2006.04904.x. [DOI] [PubMed] [Google Scholar]
- 44.Bagnard D, Vaillant C, Khuth ST, Dufay N, Lohrum M, Puschel AW, Belin MF, Bolz J, Thomasset N. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. doi: 10.1523/JNEUROSCI.21-10-03332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bondy C, Werner H, Roberts CT, Jr, LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- 46.Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Diaz-Ruiz C, Perez-Tomas R, Domingo J, Ferrer I. Immunohistochemical localization of transforming growth factor-alpha in choroid plexus of the rat and chicken. Neurosci Lett. 1993;164:44–46. doi: 10.1016/0304-3940(93)90853-d. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- 49.Johanson CE, Szmydynger-Chodobska J, Chodobski A, Baird A, McMillan P, Stopa EG. Altered formation and bulk absorption of cerebrospinal fluid in FGF-2-induced hydrocephalus. Am J Physiol. 1999;277:R263–R271. doi: 10.1152/ajpregu.1999.277.1.R263. [DOI] [PubMed] [Google Scholar]
- 50.Justicia C, Perez-Asensio FJ, Burguete MC, Salom JB, Planas AM. Administration of transforming growth factor-alpha reduces infarct volume after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2001;21:1097–1104. doi: 10.1097/00004647-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reid S, Ferretti P. Differential expression of fibroblast growth factor receptors in the developing murine choroid plexus. Brain Res Dev Brain Res. 2003;141:15–24. doi: 10.1016/s0165-3806(02)00635-1. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dziegielewska KM, Evans CA, Lorscheider FL, Malinowska DH, Mollgard K, Reynolds ML, Saunders NR. Plasma proteins in fetal sheep brain: blood-brain barrier and intracerebral distribution. J Physiol. 1981;318:239–250. doi: 10.1113/jphysiol.1981.sp013861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dziegielewska KM, Evans CA, Malinowska DH, Mollgard K, Reynolds ML, Saunders NR. Blood-cerebrospinal fluid transfer of plasma proteins during fetal development in the sheep. J Physiol. 1980;300:457–465. doi: 10.1113/jphysiol.1980.sp013172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dziegielewska KM, Knott GW, Saunders NR. The nature and composition of the internal environment of the developing brain. Cell Mol Neurobiol. 2000;20:41–56. doi: 10.1023/A:1006943926765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gato A, Martin C, Alonso MI, Martinez-Alvarez C, Moro JA. Chondroitin sulphate proteoglycan is involved in lens vesicle morphogenesis in chick embryos. Exp Eye Res. 2001;73:469–478. doi: 10.1006/exer.2001.1060. [DOI] [PubMed] [Google Scholar]
- 58.Iwama H, Ohmori S, Tsutsumi T. Detectable concentrations of urinary trypsin inhibitor in cerebrospinal fluid. J Neurosurg Anesthesiol. 2000;12:29–32. doi: 10.1097/00008506-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Back SA. Perinatal white matter injury: The changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 60.Gunn AJ, Gunn TR. Changes in risk factors for hypoxic-ischaemic seizures in term infants. Aust N Z J Obstet Gynaecol. 1997;37:36–39. doi: 10.1111/j.1479-828x.1997.tb02214.x. [DOI] [PubMed] [Google Scholar]
- 61.Stonestreet BS, Sadowska GB, McKnight AJ, Patlak C, Petersson KH. Exogenous and endogenous corticosteroids modulate blood-brain barrier development in the ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2000;279:R468–R477. doi: 10.1152/ajpregu.2000.279.2.R468. [DOI] [PubMed] [Google Scholar]
- 62.Barlow RM. The foetal sheep: Morphogenesis of the nervous system and histochemical aspects of myelination. J Comp Neurol. 1969;135:249–262. doi: 10.1002/cne.901350302. [DOI] [PubMed] [Google Scholar]
- 63.Bernhard CG, Kolmodin GM, Meyerson BA. On the prenatal development of function and structure in the somesthetic cortex of the sheep. Prog Brain Res. 1967;26:60–77. doi: 10.1016/S0079-6123(08)61419-3. [DOI] [PubMed] [Google Scholar]
- 64.Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J Child Neurol. 2006;21:582–589. doi: 10.1177/08830738060210070101. [DOI] [PubMed] [Google Scholar]
- 65.Stonestreet BS, Oen-Hsiao JM, Petersson KH, Sadowska GB, Patlak CS. Regulation of brain water during acute hyperosmolality in ovine fetuses, lambs, and adults. J Appl Physiol. 2003;94:1491–1500. doi: 10.1152/japplphysiol.00923.2002. [DOI] [PubMed] [Google Scholar]
- 66.Stonestreet BS, Petersson KH, Sadowska GB, Pettigrew KD, Patlak CS. Antenatal steroids decrease blood-brain barrier permeability in the ovine fetus. Am J Physiol. 1999;276:R283–R289. doi: 10.1152/ajpregu.1999.276.2.R283. [DOI] [PubMed] [Google Scholar]
- 67.Sysyn GD, Petersson KH, Patlak CS, Sadowska GB, Stonestreet BS. Effects of postnatal dexamethasone on blood-brain barrier permeability and brain water content in newborn lambs. Am J Physiol Regul Integr Comp Physiol. 2001;280:R547–R553. doi: 10.1152/ajpregu.2001.280.2.R547. [DOI] [PubMed] [Google Scholar]
- 68.Futamura Y, Kajikawa S, Kaga N, Shibutani Y. Protection against preterm delivery in mice by urinary trypsin inhibitor. Obstet Gynecol. 1999;93:100–108. doi: 10.1016/s0029-7844(98)00396-2. [DOI] [PubMed] [Google Scholar]
- 69.Lim YP, Josic D, Callanan H, Brown J, Hixson DC. Affinity purification and enzymatic cleavage of inter-alpha inhibitor proteins using antibody and elastase immobilized on CIM monolithic disks. J Chromatogr A. 2005;1065:39–43. doi: 10.1016/j.chroma.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 70.Kim CR, Sadowska GB, Petersson KH, Merino M, Sysyn GD, Padbury JF, Stonestreet BS. Effects of postnatal steroids on Na+/K+-ATPase activity and alpha1- and beta1-subunit protein expression in the cerebral cortex and renal cortex of newborn lambs. Reprod Fertil Dev. 2006;18:413–423. doi: 10.1071/rd05114. [DOI] [PubMed] [Google Scholar]
- 71.Malaeb SN, Sadowska GB, Stonestreet BS. Effects of maternal treatment with corticosteroids on tight junction protein expression in the cerebral cortex of the ovine fetus with and without exposure to in utero brain ischemia. Brain Res. 2007;1160:11–19. doi: 10.1016/j.brainres.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadowska GB, Malaeb SN, Stonestreet BS. Maternal glucocorticoid exposure alters tight junction protein expression in the brain of fetal sheep. Am J Physiol Heart Circ Physiol. 2010;298:H179–H188. doi: 10.1152/ajpheart.00828.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadowska GB, Stopa EG, Stonestreet BS. Ontogeny of connexin 32 and 43 expression in the cerebral cortices of ovine fetuses, newborns, and adults. Brain Res. 2009;1255:51–56. doi: 10.1016/j.brainres.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ron NP, Kazianis JA, Padbury JF, Brown CM, McGonnigal BG, Sysyn GD, Sadowska GB, Stonestreet BS. Ontogeny and the effects of corticosteroid pretreatment on aquaporin water channels in the ovine cerebral cortex. Reprod Fertil Dev. 2005;17:535–542. doi: 10.1071/rd03044. [DOI] [PubMed] [Google Scholar]
- 75.Duncan AR, Sadowska GB, Stonestreet BS. Ontogeny and the effects of exogenous and endogenous glucocorticoids on tight junction protein expression in ovine cerebral cortices. Brain Res. 2009;1303:15–25. doi: 10.1016/j.brainres.2009.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sadowska GB, Patlak CS, Petersson KH, Stonestreet BS. Effects of multiple courses of antenatal corticosteroids on blood-brain barrier permeability in the ovine fetus. J Soc Gynecol Investig. 2006;13:248–255. doi: 10.1016/j.jsgi.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 77.Stonestreet BS, Patlak CS, Pettigrew KD, Reilly CB, Cserr HF. Ontogeny of blood-brain barrier function in ovine fetuses, lambs, and adults. Am J Physiol. 1996;271:R1594–R1601. doi: 10.1152/ajpregu.1996.271.6.R1594. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen-Ba-Charvet KT, Picard-Riera N, Tessier-Lavigne M, Baron-Van Evercooren A, Sotelo C, Chedotal A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci. 2004;24:1497–1506. doi: 10.1523/JNEUROSCI.4729-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Odum L, Nielsen HW. Human protein HC (alpha 1-microglobulin) and inter-alpha-trypsin inhibitor in connective tissue. Histochem J. 1994;26:799–803. [PubMed] [Google Scholar]
- 80.Yoshida E, Sumi H, Maruyama M, Tsushima H, Matsuoka Y, Sugiki M, Mihara H. Distribution of acid stable trypsin inhibitor immunoreactivity in normal and malignant human tissues. Cancer. 1989;64:860–869. doi: 10.1002/1097-0142(19890815)64:4<860::aid-cncr2820640417>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 81.Odum L, Halkier T, Hojrup P, Schousboe I. Characterization of urinary proteinase inhibitors with segments of amino acids sequences identical to sequences of pancreatic secretory trypsin inhibitor. Int J Biochem. 1989;21:1319–1327. doi: 10.1016/0020-711x(89)90151-1. [DOI] [PubMed] [Google Scholar]
- 82.Shikimi T, Hattori K, Takaori S. Existence of a human urinary trypsin inhibitor (urinastatin)-like substance in the rat brain. Jpn J Pharmacol. 1992;60:97–103. doi: 10.1254/jjp.60.97. [DOI] [PubMed] [Google Scholar]
- 83.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koga Y, Fujita M, Tsuruta R, Koda Y, Nakahara T, Yagi T, Aoki T, Kobayashi C, Izumi T, Kasaoka S, Yuasa M, Maekawa T. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res. 2010;32:925–932. doi: 10.1179/016164110X12645013515133. [DOI] [PubMed] [Google Scholar]
- 85.Abe H, Sugino N, Matsuda T, Kanamaru T, Oyanagi S, Mori H. Effect of ulinastatin on delayed neuronal death in the gerbil hippocampus. Masui. 1996;45:38–43. [PubMed] [Google Scholar]
- 86.Monard D. Cell-derived proteases and protease inhibitors as regulators of neurite outgrowth. Trends Neurosci. 1988;11:541–544. doi: 10.1016/0166-2236(88)90182-8. [DOI] [PubMed] [Google Scholar]
- 87.Regeniter A, Kuhle J, Mehling M, Moller H, Wurster U, Freidank H, Siede WH. A modern approach to CSF analysis: pathophysiology, clinical application, proof of concept and laboratory reporting. Clin Neurol Neurosurg. 2009;111:313–318. doi: 10.1016/j.clineuro.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Saunders NR. The blood brain barrier in the foetal and newborn lamb. Ann Rech Vet. 1977;8:384–395. [PubMed] [Google Scholar]
- 89.Gato A, Martin P, Alonso MI, Martin C, Pulgar MA, Moro JA. Analysis of cerebro-spinal fluid protein composition in early developmental stages in chick embryos. J Exp Zool A Comp Exp Biol. 2004;301:280–289. doi: 10.1002/jez.a.20035. [DOI] [PubMed] [Google Scholar]
- 90.Klosovskii BN, Zhukova TP. Effect of colchicine on remote phases of growing capillaries in the brain. Arkh Patol. 1963;35:38–44. [PubMed] [Google Scholar]
- 91.Noor NM, Steer DL, Wheaton BJ, Ek CJ, Truettner JS, Dietrich WD, Dziegielewska KM, Richardson SJ, Smith AI, VandeBerg JL, Saunders NR. Age-dependent changes in the proteome following complete spinal cord transection in a postnatal South American opossum (Monodelphis domestica) PLoS One. 2011;6:e27465. doi: 10.1371/journal.pone.0027465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramey BA, Birge WJ. Development of cerebrospinal fluid and the blood-cerebrospinal fluid barrier in rabbits. Dev Biol. 1979;68:292–298. doi: 10.1016/0012-1606(79)90261-6. [DOI] [PubMed] [Google Scholar]
- 93.Saunders NR. Ontogeny of the blood-brain barrier. Exp Eye Res. 1977;25:523–550. doi: 10.1016/s0014-4835(77)80046-8. [DOI] [PubMed] [Google Scholar]
- 94.Gato A, Desmond ME. Why the embryo still matters: CSF and the neuroepithelium as interdependent regulators of embryonic brain growth, morphogenesis and histiogenesis. Dev Biol. 2009;327:263–272. doi: 10.1016/j.ydbio.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 95.Checiu I, Prelipceanu O, Popescu O. The role of the cerebrospinal fluid during embryonic development. A biochemical study. Morphol Embryol (Bucur) 1984;30:243–250. [PubMed] [Google Scholar]
- 96.Fielitz W, Esteves A, Moro R. Protein composition of cerebrospinal fluid in the developing chick embryo. Brain Res. 1984;315:111–115. doi: 10.1016/0165-3806(84)90082-8. [DOI] [PubMed] [Google Scholar]
- 97.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 98.McIntosh GH, Baghurst KI, Potter BJ, Hetzel BS. Foetal brain development in the sheep. Neuropathol Appl Neurobiol. 1979;5:103–114. doi: 10.1111/j.1365-2990.1979.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 99.Dziegielewska KM, Habgood MD, Mollgard K, Stagaard M, Saunders NR. Species-specific transfer of plasma albumin from blood into different cerebrospinal fluid compartments in the fetal sheep. J Physiol. 1991;439:215–237. doi: 10.1113/jphysiol.1991.sp018664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ek CJ, Habgood MD, Dziegielewska KM, Potter A, Saunders NR. Permeability and route of entry for lipid-insoluble molecules across brain barriers in developing Monodelphis domestica. J Physiol. 2001;536:841–853. doi: 10.1111/j.1469-7793.2001.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ek T, Pinkava M, Abrahamsson J. Ara-C fever and infections after high-dose ara-C treatment in pediatric lymphoid malignancies. J Pediatr Hematol Oncol. 2005;27:364–369. doi: 10.1097/01.mph.0000173176.33271.8f. [DOI] [PubMed] [Google Scholar]
- 102.Knott GW, Dziegielewska KM, Habgood MD, Li ZS, Saunders NR. Albumin transfer across the choroid plexus of South American opossum (Monodelphis domestica) J Physiol. 1997;499:179–194. doi: 10.1113/jphysiol.1997.sp021919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liddelow SA, Dziegielewska KM, Ek CJ, Johansson PA, Potter AM, Saunders NR. Cellular transfer of macromolecules across the developing choroid plexus of Monodelphis domestica. Eur J Neurosci. 2009;29:253–266. doi: 10.1111/j.1460-9568.2008.06571.x. [DOI] [PubMed] [Google Scholar]
- 104.Liddelow SA, Dziegielewska KM, VandeBerg JL, Noor NM, Potter AM, Saunders NR. Modification of protein transfer across blood/cerebrospinal fluid barrier in response to altered plasma protein composition during development. Eur J Neurosci. 2011;33:391–400. doi: 10.1111/j.1460-9568.2010.07509.x. [DOI] [PubMed] [Google Scholar]
- 105.Mollgard K, Saunders NR. A possible transepithelial pathway via endoplasmic reticulum in foetal sheep choroid plexus. Proc R Soc Lond B Biol Sci. 1977;199:321–326. doi: 10.1098/rspb.1977.0142. [DOI] [PubMed] [Google Scholar]
- 106.Wu R, Cui X, Lim YP, Bendelja K, Zhou M, Simms HH, Wang P. Delayed administration of human inter-alpha inhibitor proteins reduces mortality in sepsis. Crit Care Med. 2004;32:1747–1752. doi: 10.1097/01.ccm.0000132903.14121.0e. [DOI] [PubMed] [Google Scholar]
- 107.Acworth NRJ. The healthy neonatal foal: routine examinations and preventive medicine. Equine Veterinary Education. 2003;15(S6):45–49. [Google Scholar]
- 108.Crisman MV, Scarratt WK. Immunodeficiency disorders in horses. Vet Clin North Am Equine Pract. 2008;24:299–310. vi. doi: 10.1016/j.cveq.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 109.Stoffel MH, Friess AE, Hartmann SH. Ultrastructural evidence of transplacental transport of immunoglobulin G in bitches. J Reprod Fertil. 2000;118:315–326. [PubMed] [Google Scholar]
- 110.Tizard IR. Immunity in the fetus and newborn. Veterinary Immunology: An Introduction. 7th. St Louis, MO: Elsevier; 2009. pp. 221–233. [Google Scholar]